Electrospray ionization mass spectrometry (ESI-MS) has been widely used to characterize specific noncovalent biological interactions in solution, including multi-protein assemblies, protein-/peptide-ligand, and DNA-/RNA-drug complexes.1-9 In practice, ESI-MS has been used to determine binding stoichiometry,5,10-12 and to measure relative13,14 and absolute15-17 association constants (binding affinities).
While ESI-MS is clearly a powerful bioanalytical tool, this technique does have certain limitations. One of its most notable limitations is related to the tendency of biological molecules to associate nonspecifically with other biomolecules, small molecules, or ions present in solution during the ESI process.5 The formation of nonspecific noncovalent adducts with neutral or ionic species derived from buffer or impurities has been previously discussed.18,19 The formation of nonspecific noncovalent complexes may lead to misinterpretation of ESI-MS results and obscure the binding stoichiometry of specific complexes. A variety of methods have been suggested to cope with the formation of nonspecific noncovalent complexes. In particular, dialysis,20 reversed-phase high-performance liquid chromatography (HPLC),21 and size exclusion chromatography22 have been used to resolve nonspecific metal binding issues. However, these methods are limited because desalting might interfere with the protein metalation process. As a result, protein metal binding properties may not be properly reflected.
A few different metal sources that contain different counter ions have been evaluated for use in minimizing nonspecific protein-metal ion adduct formation in ESI-MS experiments. For example, Pan
In the present study, we extend the previous study by Pan
[Table 1.] Sequences and masses of zinc finger peptides used in the present study.
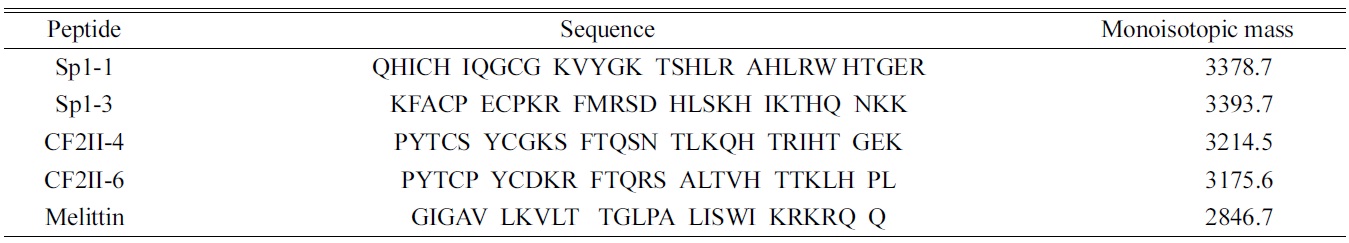
Sequences and masses of zinc finger peptides used in the present study.
Zinc finger peptides were all custom-synthesized from Peptron Inc. (Daejeon, Korea) using Fmoc solid phase chemistry. Melittin was available from Sigma (Seoul, Korea). The sequences and monoisotopic masses of zinc finger peptides used in the present study are shown in Table 1.27-29 Zinc chloride (ZnCl2), zinc acetate (Zn(CH3 COO)2), and ammonium tartrate were purchased from Sigma (Seoul, Korea). Zinc tartrate (Zn(OOC(CHOH)2 COO)) was prepared by mixing equimolar amounts of ammonium tartrate and zinc acetate. All peptide samples were dissolved at a peptide concentration of 20 μM, and the pH was adjusted to pH 7.5 using aqueous NH4OAc buffered solution.
All mass spectrometry measurements were made on an ion-trap mass spectrometer (LCQ Deca, Thermo Finnigan,
San Joes, CA) operated in the positive ion mode (needle voltage = +3.5 kV). The capillary was heated to 200 ℃ and applied potential was +10 V. The tube lens offset was maintained at +20 V. A solution of 100 mM aqueous NH4OAc buffer was infused at 3 μl min?1. Full-scan mass spectra were recorded in the range of
Three different zinc metal source compounds were added to electrospray solutions to evaluate the effects of various zinc-ion sources on the formation of zinc finger?Zn2+ complexes. Zinc chloride (ZnCl2), zinc acetate (Zn(CH3 COO)2), and zinc tartrate (Zn(OOC(CHOH)2COO)) were used as zinc metal sources.23
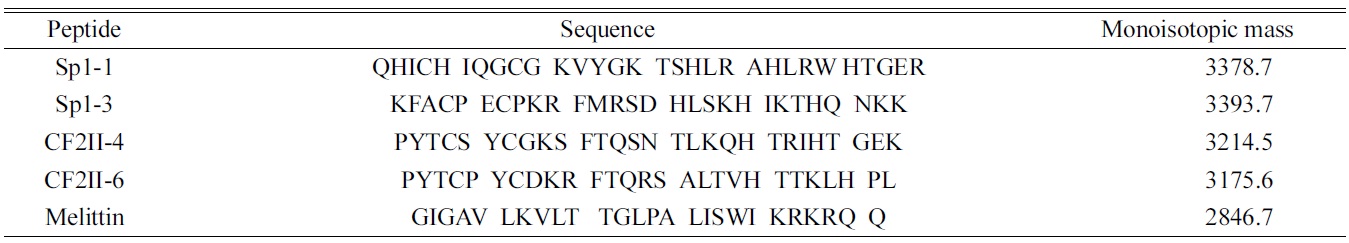
Figure 1 shows ESI mass spectra of Sp1-3, CF2II-6, and melittin obtained by co-spraying with zinc chloride, zinc acetate, and zinc tartrate, respectively. Figure 1 also shows the control ESI mass spectra obtained without any Zn2+ source. In Figure 1, only the m/z region corresponding to +3 ions is shown. Figure 1(a) and (e) show Sp1-3 and CF2II-6, respectively, when no Zn2+ source was added. Only protonated molecular ions, such as (M+3H)3+, were observed. When Zn2+ was added, Zn2+-containing molecular ions appeared. For example, when ZnCl2 was added to the ESI solution for Sp1-3 at 200 μM, the following Zn2+- adduct molecular ions were observed: (M+H+Zn2+)3+ , (M? H+2Zn2+)3+, (M?3H+3Zn2+)3+, (M?5H+4Zn2+)3+, and (M? 7H+5Zn2+)3+ (see Figure 1(b)). Sp1-3 zinc finger peptide ions with a single Zn2+, i.e., (M+H+Zn2+)3+, reflect the native zinc finger metal binding stoichiometry. In addition to these ions, Zn2+-adducts with multiple Zn2+ ions were observed: (M?H+2Zn2+)3+, (M?3H+3Zn2+)3+, (M?5H+ 4Zn2+)3+, and (M?7H+5Zn2+)3+. Among many Zn2+ ions contained in [M+(3?2n)H+nZn2+]3+ molecular ions, one structural Zn2+ ion is very likely to coordinate in a nativelike metal ion binding site while the other Zn2+ ions are likely to be adducted. In other words, zinc finger peptide ions with multiple Zn2+ ions are likely to form nonspecific complexes, which should be avoided in this experiment. The addition of Zn(CH3COO)2 yielded very similar results. As shown in Figure 1(c), Zn2+-adducts with multiple Zn2+ ions were observed. In contrast, when Zn(tartrate) was added at 200 μM, Sp1-3 zinc finger peptide ions with a single Zn2+, i.e., (M+H+Zn2+)3+ , were dominant with a low abundance of (M?H+2Zn2+)3+ ions containing two Zn2+ ions (see Figure 1(d)). This result clearly indicates that when Zn(tartrate) was used, the formation of nonspecific complexes with multiple Zn2+ ions was significantly reduced, consistent with the previous study by Pan
For CF2II-6, the above-described Zn2+-adduct formation tendency was also observed. ESI solutions prepared with either ZnCl2 or Zn(CH3COO)2 produced significant amounts of nonspecific zinc finger?Zn2+ complexes (see Figure 1(f) and (g)). Specifically, the ESI solution with ZnCl2 showed ESI mass distribution of (M+3H)3+, (M+H+Zn2+)3+, (M?H+2Zn2+)3+, (M?3H+Zn2+)3+, and (M?5H+4Zn2+)3+. The ESI solution with Zn(CH3COO)2 yielded a distribution with (M+3H)3+ , (M+H+Zn2+)3+, and (M?H+2Zn2+)3+. As described above, zinc finger?Zn2+ complexes with multiple zinc ions are simple Zn2+ adducts that do not reflect the stoichiometry of native zinc finger peptides. ESI-MS results obtained with Zn(CH3COO)2 were very similar to those obtained with ZnCl2. However, for Zn(CH3COO)2, the production of (M?3H+3Zn2+)3+ and (M?5H+4Zn2+)3+ was completely quenched. When Zn(tartrate) was used as a metal source, the generation of nonspecific zinc finger?Zn2+ complexes was not observed at all. As shown in Figure 1(h), only (M+3H)3+ and (M+H+Zn2+)3+ were observed, indicating that the formation of nonspecific complexes with multiple Zn2+ ions was completely inhibited. It is also noteworthy to note that when Zn2+ was added, Sp1-3 did not show any (M+3H)3+, while CF2II-6 showed abundant (M+3H)3+ peaks. This different complex formation behavior may be due to differences in the Zn2+ binding constants of the two peptides. In our unpublished data, the Zn2+ binding constant of Sp1-3 was higher than that of CF2II-6 by approximately two orders of magnitude.
To confirm whether Zn(tartrate) generally reduces the formation of nonspecific zinc finger?Zn2+ complexes, the same approach was repeated for other peptides, such as Sp1- 1 and CF2II-4. The formation of nonspecific complexes was significantly reduced (spectra not shown) for the other peptides. We also performed another control experiment with melittin, which has a mass similar to those of other zinc finger peptides but does not have any Zn2+ binding properties. Figure 1 (i)-(l) shows the resulting ESI mass spectra. As expected, the ESI solution with Zn(tartrate) did not produce any zinc finger?Zn2+ complex, while the addition of ZnCl2 or Zn(CH3COO)2 caused the formation of zinc finger?Zn2+ complexes with multiple Zn2+ ion complexes. These control experiments clearly suggest that Zn(tartrate) is an appropriate zinc ion source that minimizes the formation of nonspecific zinc finger?Zn2+ complexes.
A detailed mechanistic study on why tartrate reduces the formation of nonspecific complexes between metal binding peptides and metal ions is still required.23 However, the relatively high Zn2+ binding affinity of tartrate was previously suggested to play a certain role in reducing nonspecific complexes. Acetate ion, which has a relatively higher Zn2+ binding ability than chloride ion, was shown to produce less nonspecific complexes than chloride ions did (see Figure 1).