Among the perovskite-related compounds, which is represented by a general formula of An+1BnO3n+1, when n=∞ we get ABO3, the perovskite system [1,2]. If n=1 then, the system can be represented as A2BO4. This is generally called as K2NiF4 system. Many compounds, generally oxides or halogenides, belong to this structure. The characteristics of perovskite-related iron oxides are known to change with respect to various heating conditions [3] such as heating atmosphere (in air, in oxygen or in vacuum), maximum heating temperature, and cooling conditions like slowly cooling or quenching.
Perovskite-type or K2NiF4-type oxides are known for the presence of mixed electronic and oxygen-ionic conductivity and mixed valencies of the transition metals in them. They have been of great interest for electrochemical applications, such as dense ceramic membranes for oxygen separation, electrodes of solid oxide fuel cells (SOFCs) and partial oxidation of hydrocarbons [4-7]. The K2NiF4-type structure, in the first place was described by Balz [8] and Ruddelsden [9]. They can be explained as a series of layers of tetragonally distorted NiF6 octahedral, and the K+ ions are located in 9-coordinated sites between the layers. If the transition metals such as Fe, Cr and Co are introduced in the octahedral then, the K2NiF4-type compounds show the so-called 2-dimensional (2-D) characters [10], which are different from those of perovskites [11,12]. Before this study, (Sr1-xBax)LaFe3+1-τFe4+τO4-y system [13] was known as a good additive for electrical conducting inorganic adhesives (ECIA), but later it was found out to have some faults such as irreversibility in gaining the oxygen when the temperature decreased from high temperature. However, the (Sr1-xBax)NdFe3+1-τFe4+τO4-y system did not show any oversensitivity to oxygen. In this study, synthesis and heat-treatments of the (Sr1-xBax)NdFe3+1-τFe4+τO4-y system (x=0.0, 0.1, 0.2 and 0.3) in air were performed. And crystallographic structures, changes in Fe3+ contents and in oxygen contents with temperature, electrical conductivities were studied and discussed. Finally some basic tests for applicability as additives for ECIA were executed.
The solid solutions of the (Sr1-xBax)NdFe3+1-τFe4+τO4-y system with the composition of x=0.0(SBN-0), 0.1(SBN-1), 0.2(SBN-2) and 0.3(SBN-3) were prepared using the traditional direct-reaction of the mixtures of starting materials. After the calculation of the stoichiometries of each composition for 0.011mole and mixing the starting materials such as SrCO3 (Aldrich Chemical Company 99.9%), BaCO3 (Aldrich Chemical Company 99.99%), Nd2O3(Aldrich Chemical Company 99.99%) and Fe2O3( Junsei, 99.9%) of the appropriate proportions, the four samples were synthesized at 1,473 K in air for 12 hr. The pressed pellets (diameter: 10 mm, thickness : 1.5-2 mm) with each composition were fabricated under a pressure of 2 ton/cm2 for 5minutes and sintered in air at the same temperature of a synthesis process. And finally all the pellets and powder samples of each composition were heat-treated in air at 1,073 K for 24 h and then cooled using a reactor. After the synthesis process, the crystallography of each prepared sample was studied by the X-ray powder diffraction (XRD, PW1710 [Philips, Almelo, Netherlands]) method. X-ray diffraction patterns of the four compositions were obtained using monochromatized CuKα radiation in the 2 θ range of 20-60°.
In Mohr salt analysis, 0.1g of each sample was dissolved in an acidic medium of HCl and H2SO4 with an excess of Mohr salt. It was then, back-titrated with 0.1N-K2Cr2O7 solution using C24H20O6N2S2Ba (97%, Junsei Chemical Co. Ltd.) as an indicator. The amount of Fe4+ ion (τ) was calculated from the amount of remaining Fe2+ ions according to the following equation.
τ Fe4+ + Fe2+(excess) → 2τ Fe3+ + (1-τ) Fe2+ (residual)
Finally the y values, the concentrations of anionic vacancy, were deduced from the chemical formula of (Sr1-xBax)NdFe3+1-τFe4+τO4-y considering the electro-neutrality condition.
TG/DTA analyses using TG/DTA 220 (Seiko Instruments Inc., Chiba, Japan) were carried out for each sample in air (303-1,073 K). The TG data (weight change) were measured with the heating rate of 20 K/min at the intervals of 10 K and then, converted into 4-y values by the application of the related formulas [3,12].
The electrical conductivities of the four samples were measured in the temperature range of about 293-1,173 K under atmospheric air pressure. Applying the four point probe technique,independent measurements of the currents with constant voltage were performed using model 236 SMU(Keithley Co., Cleveland, Ohio, U.S.A.) at the temperature intervals of 50 K. The instruments for the four probe measurements were made using quartz and platinum wire for special usage such as high temperature conductivity at various oxygen pressures. Considering the chemical and physical properties of each sample, ECIAs were manufactured by mixing the four samples (80 wt%) with an inorganic adhesive solution of AB11[14]. It was composed of silicates, alumina, magnesia and small amount of organic adhesives. For testing the adhesive powers, samples were prepared by attaching two pieces of glass plate (15 mm × 30 mm × 3 mm) painted with each ECIA (15 mm × 15 mm) and cured at 433 K for 2 h and then 493 K for 1 h. With increasing weights from 0.5 kg up to 2 kg, the adhesive powers were examined by applying the KS standard method of M3734 for tensile strength. And another experiment to measure the conductivities of each ECIA painted on a piece of glass with the area of 150 mm2 (10 mm × 15 mm) were performed by the application of the standard 4-probe DC method after heat-treatments at 473 K for 2 h.
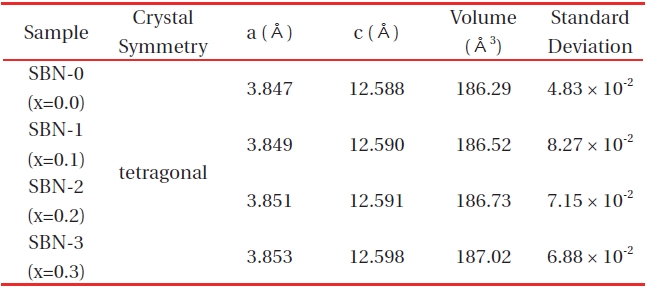
[Table 1.] X-ray powder diffraction data of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system.
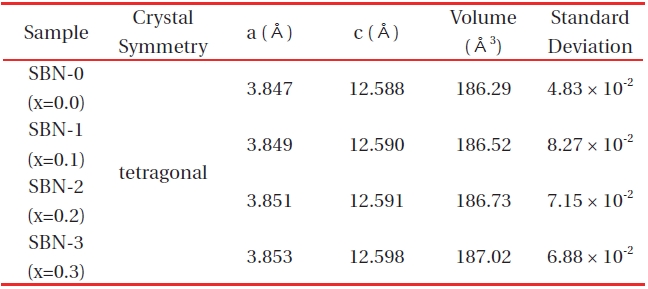
X-ray powder diffraction data of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system.
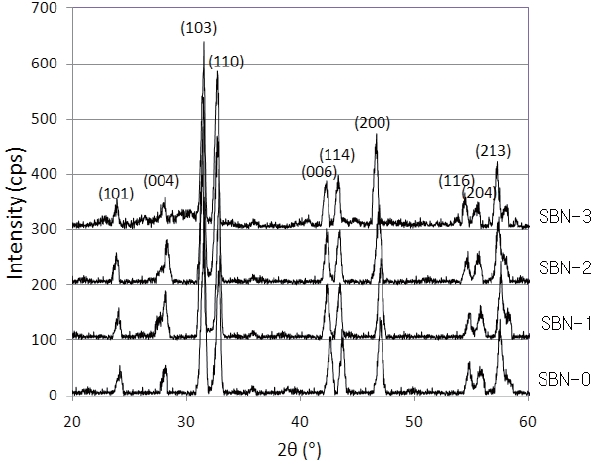
The solid solutions of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system (x=0.0 (SBN-0), 0.1 (SBN-1), 0.2 (SBN-2) and 0.3 (SBN-3)) were synthesized by heating the mixtures of the reactants at 1,473 K in air. Figure 1 shows the X-Ray diffractograms of the four samples.
After a reasonable indexation that refers to the SrNdFeO4 system (JCPDS #25-0903), the four samples were confirmed to have typical K2NiF4 structures (tetragonal symmetry, I4/mmm). Especially the XRD data (Table 1) of SBN-0 (a=3.847 Å, c=12.588 Å) almost coincided with those of SrNdFeO4 (a=3.846 Å, c=12.59 Å).
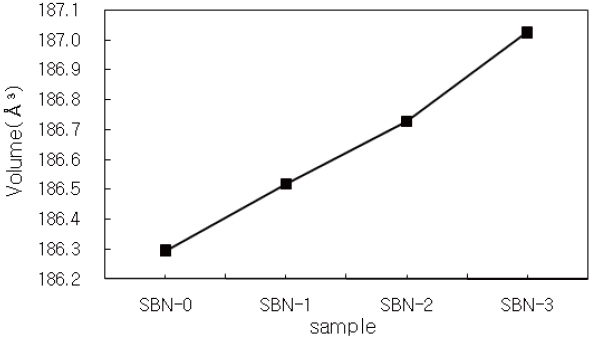
From the lattice parameters, it was found out that the partial replacement of Sr by Ba resulted in a volume expansion (Fig. 2) from 186.29 Å 3(SBN-0) to 187.02 Å 3(SBN-3). This unit cell volume change [15] is natural when we consider the Vegard's law, i.e. the partial substitution of Ba ions having a larger ionic radius of 1.75 Å for the smaller Sr (r=1.58 Å) sites may cause an increase in the unit cell volumes. On the contrary in BaxLa1-xFeO3-x/2 system [16], it was observed that the partial substitution of the smaller La3+ ion for the larger Ba2+ sites caused the unit cell volume contraction.
For this kind of tendency in perovskite-related compounds, there are three factors ruling volume change; (1) variation in mole fraction of a larger ion(Ba2+ in this study), (2) variation of Fe4+ mole fraction, and (3) formation of oxygen deficiency or excess of oxygen. In this system, oxygen content gradually increased along with the x values (Table 2). So, it can be concluded that the factors (1) and (3) predominates in the change of unit cell volume.
The main purpose of this study is to investigate the mixed valency of iron, thermal properties, electrical conductivities and
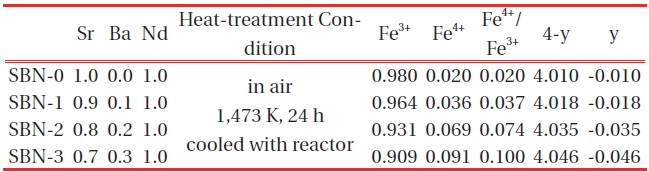
[Table 2.] Chemical analysis (mohr salt analysis) data of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system.
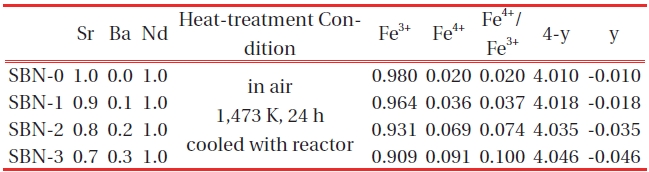
Chemical analysis (mohr salt analysis) data of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system.
finally applicability as electrical conducting adhesives of (Sr1-xBax) NdFe3+1-τFe4+τO4-y system. It is well-known that the introduction of oxygen vacancies and/or the coexistence of Fe3+/Fe4+ can give rise to a substantial change in the electronic state. This is clearly seen in the perovskite-related materials [17]. So it is very important to study the mixed valence states of transition metal (Fe).
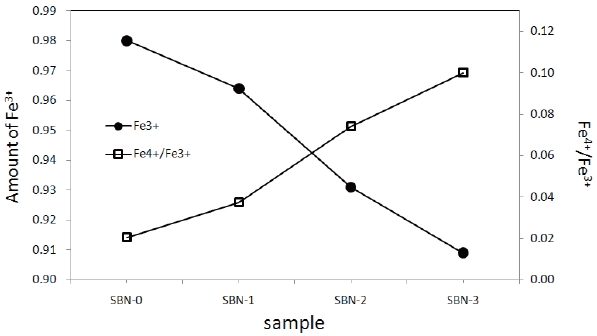
With the increase of Ba, the amounts of Fe4+(τ) and oxygen (4-y) were also increased (Fig. 3, Table 2) simultaneously, and all the sample s turned out to have excessive oxygen (Table 2), i.e. 4-y>4.0. Sr1+xDy1-xFeO4-y system [18] showed excessive oxygen content only for the compositions x=0.00, and the rest of the samples with x>0.25 were oxygen deficient (when x=0.25, 4-y=3.95). In such a system, + charge deficiency caused by the increase of Sr was compensated mainly by the formation of oxygen deficiencies (4-y<4.0) rather than by Fe4+ formation. However, in this system the oxidation state of Ba ion is the same as that of Sr. As a result, the substitution of Ba has no effects on charge differences.
So, it can be inferred that a slight increase in 4-y values was due to the heating condition of the ordinary oxygen pressure and relatively slow cooling rate.
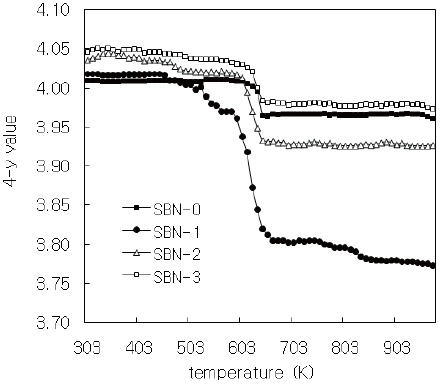
Figure 4 is the TG/DTA data in which all the weight data were converted into 4-y values and we assume that the weight change was caused only by the oxygen amounts’ variation with respect
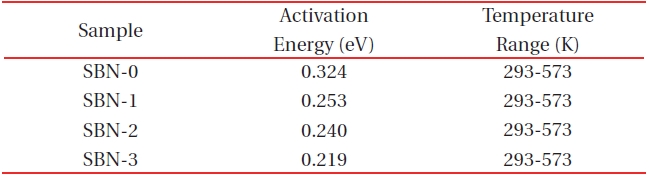
[Table 3.] Activation energies from the electrical conductivity data.
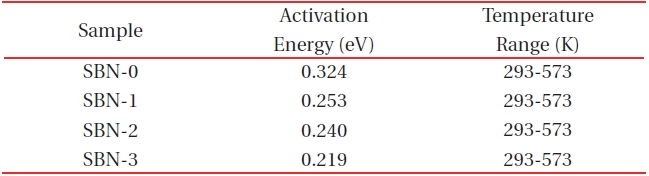
Activation energies from the electrical conductivity data.
to temperature change.
Around 600 K all the samples commenced to decrease (Fig. 4) in weights. This means the outgoing of oxygen and this temperature region almost coincided with that of the break point conductivity data (Fig. 5) for the four samples.
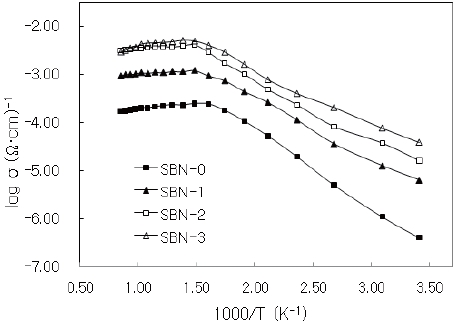
Electrical conductivity (σ) data [19] obtained from the temperature range of 293-1,173 K in air shows that SBN-0 has the lowestσ values among the four samples at a constant temperature. For example, at 323 K (1,000/T=3.10), each sample shows an increase in the conductivity values and a decrease in the activation energies (Table 3) in the order of SBN-0→SBN-1→SBN-2→SBN-3. It has been well known that in the perovskite-related materials as the Fe 3+/Fe4+ ratios approach 1, we get the largest conductivity value (σ) of all the samples [20,21]. In this study a similar tendency was observed; while Fe3+/Fe4+ ratio of SBN-0 is 0.02 (log σ=-6.39 (ohm-1cm-1) at 293 K) and that of SBN-3 is 0.10 (log σ=-4.11 (ohm-1cm-1) at 293 K). In other words, with an increase in Ba amounts from x=0.0 to x=0.3, the conductivity value increased by more than 2 order (Fig. 5).
In perovskite-related materials containing mixed valenced transition metal ions, their conduction is understood to be as hopping mechanism [15] and not as tunneling mechanism.
The conductivities of the four samples showed maxima [22] in
[Table 4.] Adhesive power and electrical conductivity for each ECIA specimen.
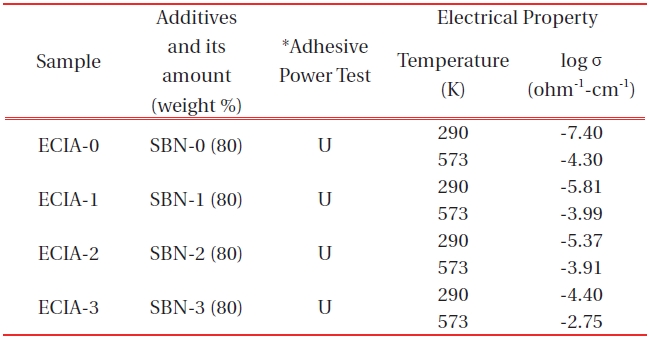
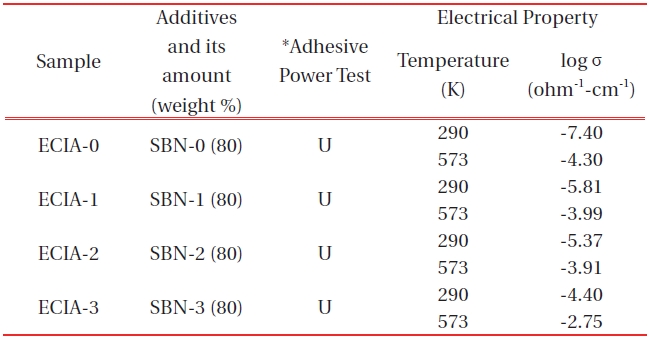
Adhesive power and electrical conductivity for each ECIA specimen.
conductivity at about 600 K. As discussed before, the decrease in conductivity above 600 K may be attributed to the loss of oxygen and the corresponding increase in Fe3+ content over the Fe4+ content may result in a reduced number of electron hole sites [23].
Considering the applicability of the additives for ECIA, a candidate material should possess certain characteristics such as stability in alkaline bath, linearity of conductivity data with temperature, temperature-insensitivity to oxygen content and the reversibility of oxygen in the cooling process when heated up to a high temperature. By mixing an appropriate amount (80 weight %) of each sample with an inorganic adhesive of AB11(Table 4), four electrical conducting inorganic adhesives (ECIA) were prepared. All the four specimens(15 mm ×15 mm) had a tensile strength that was strong enough to endure the force of 19.6 N (2 kg of weights, KS standard method of M3734)). The adhesive power tests were performed only up to 2 kg because when the weights were over 2 kg, one of the two pieces of glass broke. The order of conductivity values which was observed in the conductivity measurements of SBN pellets was also maintained for all the ECIAs; ECIA-0→ECIA-1→ECIA-2→ ECIA-3 (Table 4), i.e. at 290 K, log σ value of ECIA-0 was -7.40 (ohm-1cm-1) and that of ECIA-3 was -4.40 (ohm-1cm-1).
Conclusively the adhesive properties of ECIA made of SBN system have been verified to be satisfactory due to the following aspects; excellent linearities of electrical conductivity values with temperature change, considerably good adhesive power, stability in water and alkaline bath, and reversibility of oxygen in cooling process from high temperature.
The solid solutions of (Sr1-xBax)NdFe3+1-τFe4+τO4-y system having the composition of x=0.0(SBN-0), 0.1(SBN-1), 0.2(SBN-2) and 0.3(SBN-3)) were prepared at 1473K in air. XRD study showed that all the four solid solutions possessed tetragonal symmetries (SBN-0 : a=3.847 Å, c=12.588 Å, SBN-1 : a=3.849 Å, c=12.590 Å, SBN-2 : a=3.851 Å, c=12.591 Å and SBN-3 : a=3.853Å, c=12.598 Å) and the unit cell volumes increased along with the x values. The nonstoichiometric chemical formulas of the four samples determined by Mohr salt analysis were as follows; SBN-0 : Sr1.0Nd1.0Fe3+0.980Fe4+0.020O4.010, SBN-1 : Sr0.9Ba0.1Nd1.0Fe3+0.964Fe4+0.036O4.018, SBN-2 : Sr0.8Ba0.2Nd1.0Fe3+0.931Fe4+0.069O4.035, and SBN-3 : Sr0.7Ba0.3Nd1.0Fe3+0.909Fe4+0.091 O4.046. With the increase of Ba content, the amounts of Fe4+(τ) and oxygen (4-y) were increased, and 4-y values were over 4.0 (oxygen excessive). All the samples started to lose their oxygen at about 600 K and this temperature region of each sample almost coincided with the maximum of conductivity values. At constant temperature, the order of conductivity values was SBN-0 → SBN-1 → SBN-2 → SBN-3. The tensile strengths of the four ECIA specimens fabricated with the inorganic adhesive of AB11 were sufficiently large enough to bear the force of 19.6N. The conductivity order of ECIAs on pieces of glass (10 mm ×15 mm) at a constant temperature (290 K and 573 K) was ECIA-0 → ECIA-1 → ECIA-2 → ECIA-3 and the order wholly coincided with that of the bulk samples. The (Sr1-xBax)NdFe3+1-τFe4+τO4-y system was ascertained to be a satisfactory material as an additive for the electrical conducting inorganic adhesives.