Langasite La3Ga5SiO14 (LGS) group crystals are now attracting researcher’s attention on the properties of high temperature performance, because they exhibit no curie point and no phase transition up until a melting point of around 1,500℃ and also no pyroelectricity depending on the point group 32, which is the same as quartz [1]. The properties are applied to the direct measuring of the combustion (burning) pressure sensor at the high temperature and the high pressure in combusting engines [2]. The piezoelectric constant of langasite is a superior sensitivity characteristic k ~ 15-25 approximately 3 times that of quartz crystal k ~ 7% [1]. On the other hand, langasite has been expected for surface acoustic wave (SAW) filter, because of low loss (high Q) and near zero temperature coefficient of frequency (TCf).
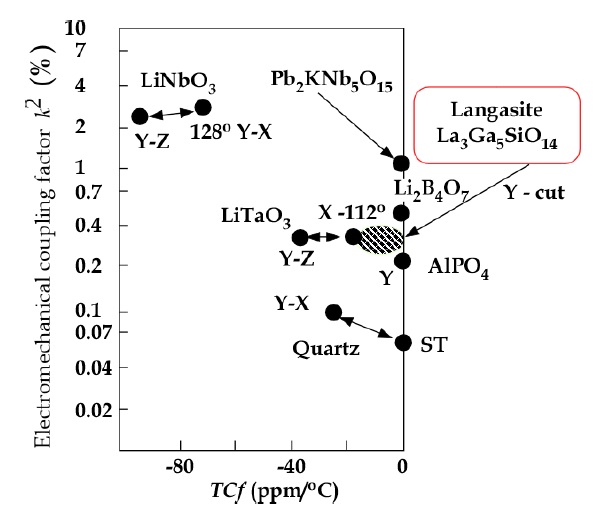
The Q values of LGS and La3Ga5.5Nb0.5O14 (LGN) are 30,000-40,000 and 60,000-120,000, respectively [1]. These values are higher than that of LiTaO3 5,000, but lower than that of quartz 100,000- 200,000. Figure 1 shows the electromechanical coupling factor k2 v.s. temperature coefficient of frequency regarding piezoelectric materials. Though some crystals are located around near zero TCf, LiTaO3 crystal with wide-band does not show near zero TCf [1]. However, the LiTaO3 SAW filter made by Yamaju Ceramics is used by roughly 60% of the world. Though AlPO4 crystals with near zero TCf and a high electromechanical coupling factor K2 are expected for SAW filter, they could not grow as good crystals because of twining during crystal growth. Li2B4O7 crystal shows deliquescent and the growing speed is too slow. For langasite, the growing of crystals is easy and processing is superior because of their good properties, and langasite group compounds have fabricated more than 150 different compositions.
We presented three component compounds such as R3Ga5SiO14 (R = La, Pr and Nd) [3-5], which are analyzed by single crystal
X-ray diffraction. The crystal structure originally presented by Belokoneva et al. [6] and Mill et al. [7] is shown in Fig. 2. The structure has four oxygen polyhedra including cations: A-site of decahedron including La3+, B-site of octahedron with Ga3+, and two C-and D-sites of tetrahedra with Ga3+ and Si4+. The crystal structural formulae are shown as A3BC3D2O14. These sites include many kinds of ions, which create compounds of more than 150 species.
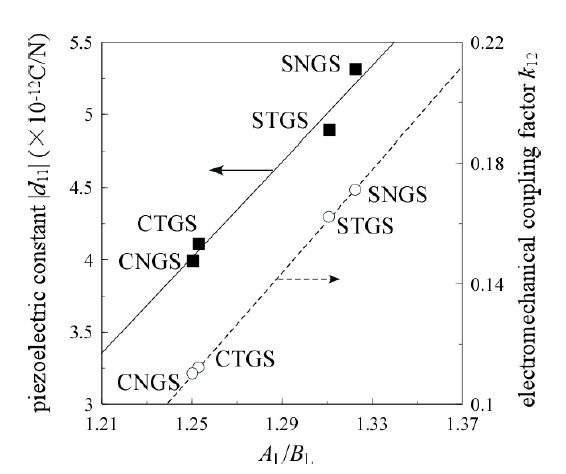
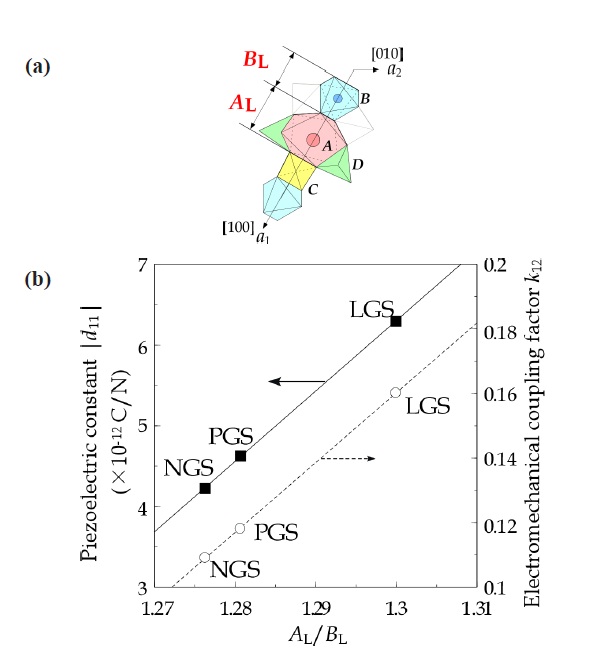
Furthermore, the relationship between the deformation of decahedron (A-site) and piezoelectric modulus d11 was shown in Fig. 3 [8], and the piezoelectric constant d11 and the electromechanical coupling factor k12 are shown as a function of AL/BL as shown in Fig. 4. Also, we presented the mechanism of piezoelectric properties based on the crystal structure as shown in Fig. 5 [4,5,8]. The structure has an open-space on the row along the a-axis. It works as a buffer area and forms a net dipole moment according to moving the center of a mass regarding positive and negative charges, and produces dipole moments.
In this work, we investigated the crystal structures of four component langasites: Sr3TaGa3Si2O14 (STGS), Ca3TaGa3Si2O14 (CTGS), Sr3NbGa3Si2O14 (SNGS), and Ca3NbGa3Si2O14 (CNGS) and clarified the effects for A-site substitution Sr for Ca on STGS/SNGS and CTGS/CNGS, and for B-site substitution Ta for Nb on STGS/CTGS and SNGS/CNGS. Moreover, we clarified the generation mechanism of the piezoelectricity and the reason why LGS has the best piezoelectric properties.
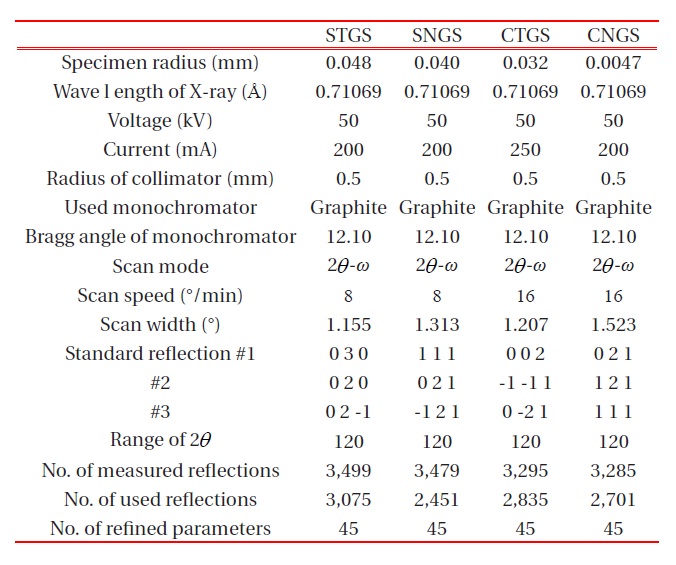
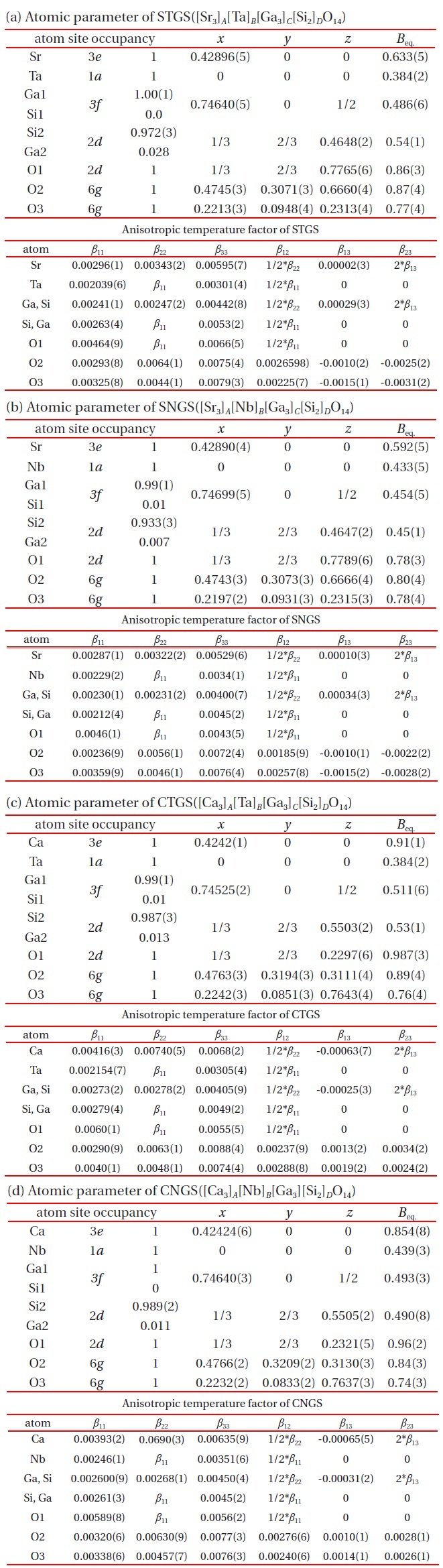
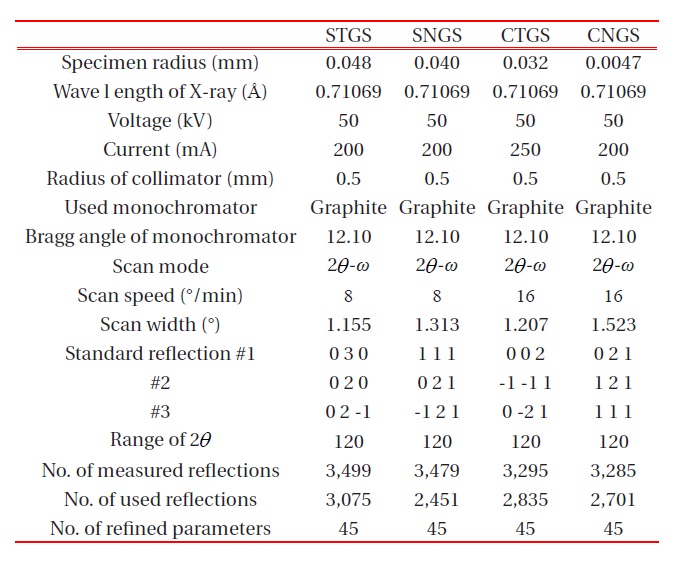
The crystals used for crystal structure analysis were grown by the Czochralski method. The conditions of the growth are shown in a previous paper [9]. A single crystal cut from the grown crystal was ground into a sphere and used for the single-crystal structure analysis. X-ray intensity data was obtained with MoKa radiation (λ = 0.71069 A ) on a four-circle diffractometer using a graphite monochromator as shown in Table 1. After Lp and absorption correction, the refinement of the crystal structure was performed by the full-matrix least-squares program RADY [10]. The site occupancies for both C- and D-site Ga: Si were obtained from multiplicity g determined by the linear constraint as follows for C-site (position : 3f, multiplicity = 1/2) :
and for D-site (position : 2d, multiplicity = 1/3) :
Beq. values were calculated from anisotropic temperature coefficients refined as follows:
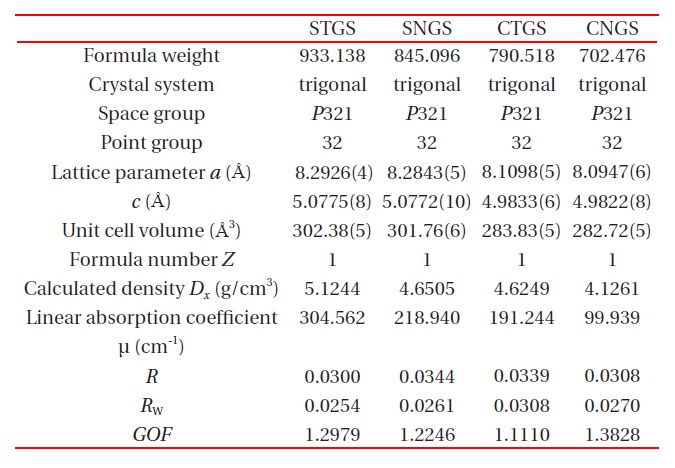
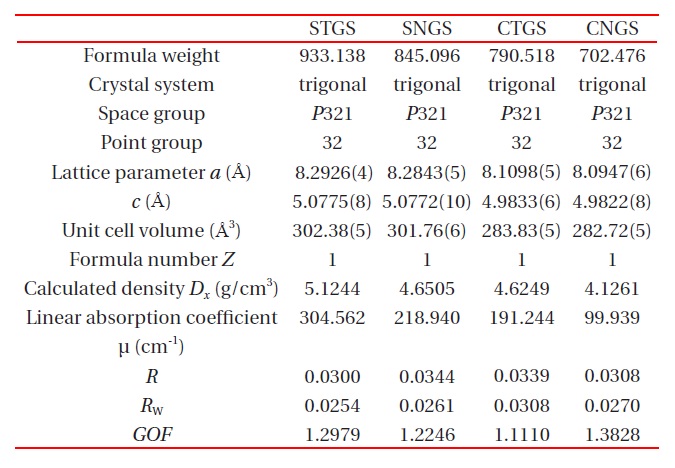
The unit cell parameters were refined and determined by a least-square calculation of twenty values for 2θ between 70° ≤ 2θ ≤ 80° . The crystallographic data and experimental condition are shown in Table 2.
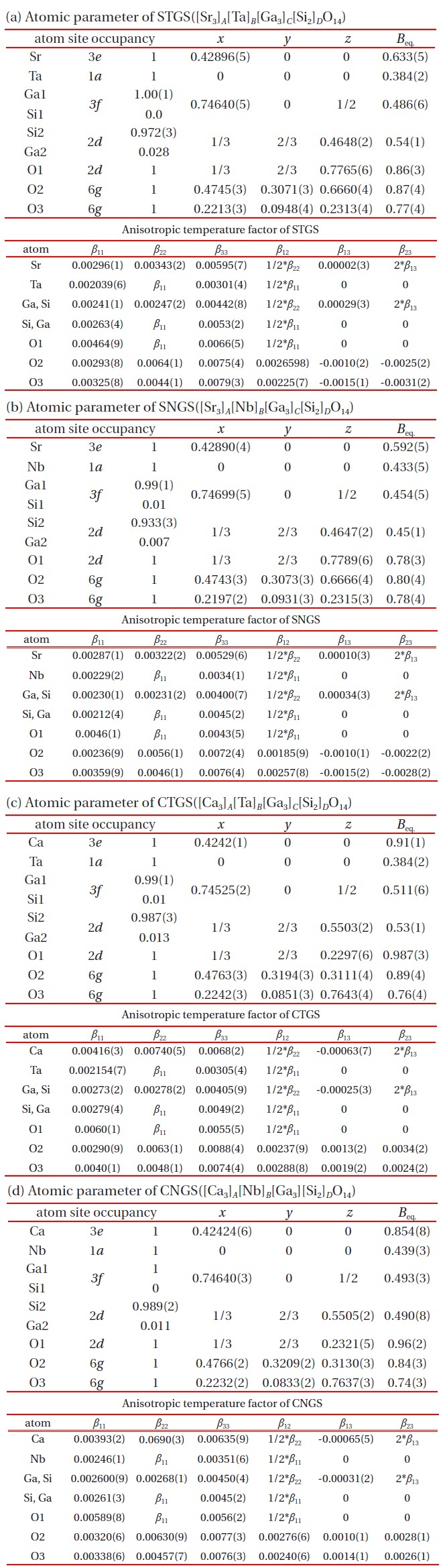
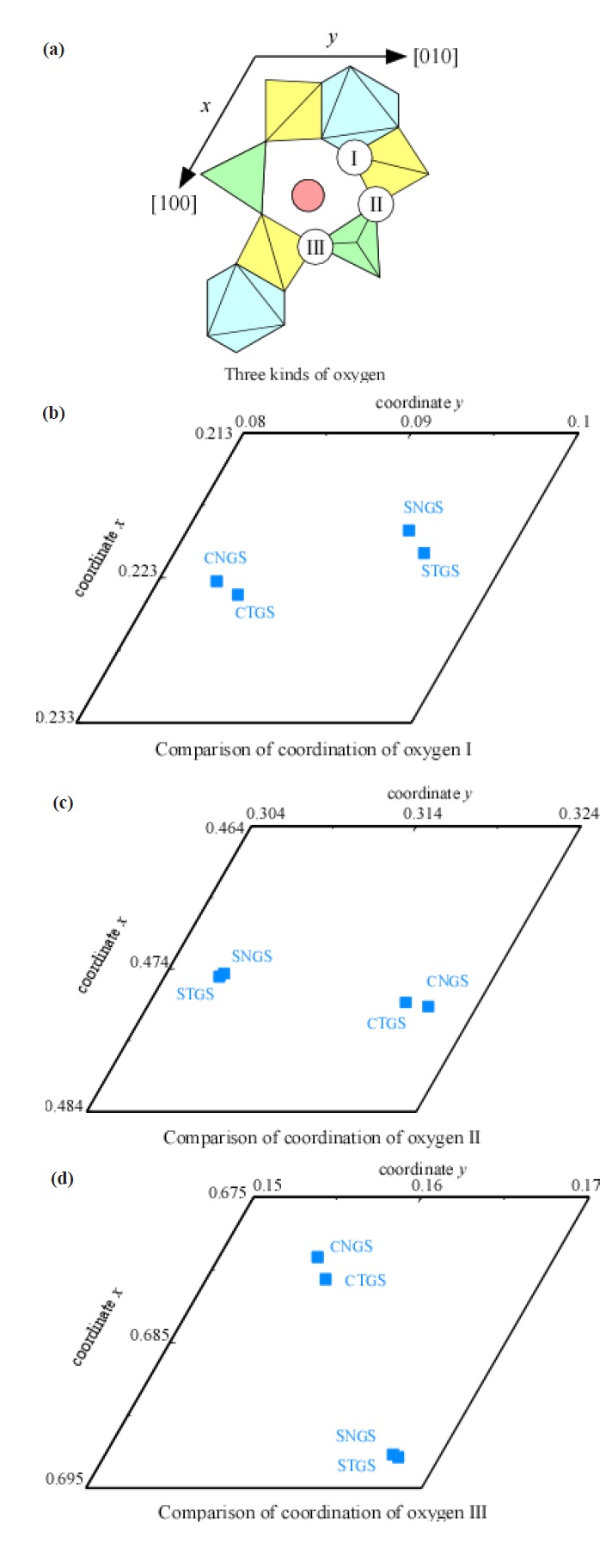
The atomic coordinates of STGS, SNGS, CTGS, and CNGS are presented in Table 3(a) to (d), respectively. These crystal structures are converged successfully from 3 to 3.4 % for reliability factor R refining as a trigonal system and space group P321, as shown in Table 2. The lattice parameters of CTGS/CNGS are converged to a = 8.10 and c = 4.98 A, and those of STGS/SNGS a = 8.29 and c = 5.08 A. The lattice parameters of Sr-langasite are larger than Ca-langasite, because the ionic size of Sr (r = 1.26 A ) is larger than that of Ca (r = 1.12 A). Those of Ta- and Nd-analogy are nearly the same, due to the similar ionic size of Ta and Nb ions (r = 0.64 A ). C- and D-sites are occupied mostly by Ga and Si, respectively, as shown in Table 3. The equivalent temperature factors Beq. of B-site (around 0.4 A2) and oxygen ions (around 0.9 A2) are reasonable values. Those of A-site (around 0.9 A2 for Calangasite and 0.6 for Sr-langasite) are a little large according to a large size polyhedron with a fluctuation of cations, and as the ionic size of Ca is small, the fluctuation becomes large. Further, C- and D-sites (around 0.5 A2) are also a little large because these sites are occupied by two cations Ga and Si. Especially, the D-site depends on the amount of site occupancies as shown in Fig. 6. Differences of oxygen positions (I, II, and III) on A-polyhedron
in regards to four component langasites are shown in Fig. 7. We brought out two effects for substitutions of A- and B-cations. The first one is A-site substitution Sr for Ca on STGS/SNGS and CTGS/CNGS. The second one is B-site substitution Ta for Nb on
STGS/ CTGS and SNGS/ CNGS. In the case of the first one, shifts of oxygen ions are large: oxygen I and III shift towards expanding, and oxygen II shift towards shrinking, as shown in Fig. 8(a). As a result, A-polyhedron expands toward [100] and shrinks toward [120]. In the case of the second one, shifts of oxygen ions in B-octahedron are small as seen in Fig. 7, because the ionic size difference between Nb and Ta is small. The oxygen ions being equivalent rotate around the 3-fold axis as shown in Fig. 8(b). As a result, BL becomes large and AL does not change. The ionic radius of Ta is empirically larger than that of Nd. We present the piezoelectric constant d11 and the electromechanical coupling factor k12 of 4-component langasites as a function of AL/ BL as shown in Fig. 9. Expansion toward [100] obtained in the case of A-site substitution by a large ion brings about large value regarding AL/ BL. So, Sr-substituted langasites have large d11 and k12 . In the case of B-site substitution, as AL does not change, the property differences between Nb and Ta are not significant.
Four component langasite STGS, SNGS, CTGS, and CNGS crystal structures are analyzed by X-ray single crystal structure analysis. The crystal data such as trigonal, P321, lattice constants, as well as the atomic parameters and anisotropic temperature coefficients for each cation are presented by precise analysis of the reliability factor R of approximately 3%. The values for d11 and k12 are presented as a function of the AL/BL ratio, which show a complete linear relationship. Two effects for substitutions A- and B-site cations are presented: the substitution Sr for Ca brings expansion toward [100] and an increase of AL/BL enlarges d11 and k12. On the other hand, as the substitution Ta for Nb brings little change, the property values are similar.




![Crystal structure of langasite. (a) and (b) are viewed from [001] and [120], respectively. (c) four kinds of cation polyhedra.](http://oak.go.kr/repository/journal/11398/E1TEAO_2012_v13n4_171_f002.jpg)
![(a) Size AL1 and AL2 of A-polyhedron along [100] and [120], respectively, (b) the ratio AL1/AL2 and piezoelectric modulus | d11| as a function of the ionic radius of R.](http://oak.go.kr/repository/journal/11398/E1TEAO_2012_v13n4_171_f003.jpg)
![Origin of piezoelectricity on langasite projected from [120]. Position X is the center of oxygen atoms on A-polyhedron. Under pressure, ML does not change and NL shrinks because of the role of openspace as a damper.](http://oak.go.kr/repository/journal/11398/E1TEAO_2012_v13n4_171_f005.jpg)
![(a) Deformation of A-polyhedron by substitution Sr for Ca. As a result, A-polyhedron expands toward [100] and shrinks toward [120], (b) deformation of B-polyhedron by substitution Ta for Nd. The equivalent oxygen ions rotate around the 3-fold axis, and the BL length becomes large.](http://oak.go.kr/repository/journal/11398/E1TEAO_2012_v13n4_171_f008.jpg)