In order to synthesize LED phosphor materials, we have applied three novel synthesis techniques, “melt synthesis”, “fluidized bed synthesis” and “vapor-solid hybrid synthesis”, in contrast with the conventional solid state reaction technique. These synthesis techniques are also a general and powerful tool for rapid screening and improvements of new phosphor materials.
The conventional mercury-discharged fluorescent lamp illuminates our surroundings, and helps our comfortable life. Many laws in the world restrict the use of hazardous substances in the manufacture of electrical and electronic equipment, such as lead, mercury, cadmium and hexavalent chromium. Although a mercury-discharge based fluorescent lamp is not a highly restricted object at present, mercury based lighting will be prohibited in the near future. White LED is the most promising candidate for new lighting systems in the near future. However, the luminance of the present white LED is not enough for interior lighting. Therefore, developments of more efficient LED phosphors are of prime importance. A bulk phosphor of over ten-micrometers diameter has usually been used in the applications of LED phosphors. In order to synthesize well-grown and well-crystallized LED phosphor materials, we have applied three novel synthesis techniques: “melt synthesis”, “fluidized bed synthesis” and “vapor-solid hybrid synthesis”. To the best of our knowledge, no such new processes for the production of wellgrown LED phosphors have been found to date.
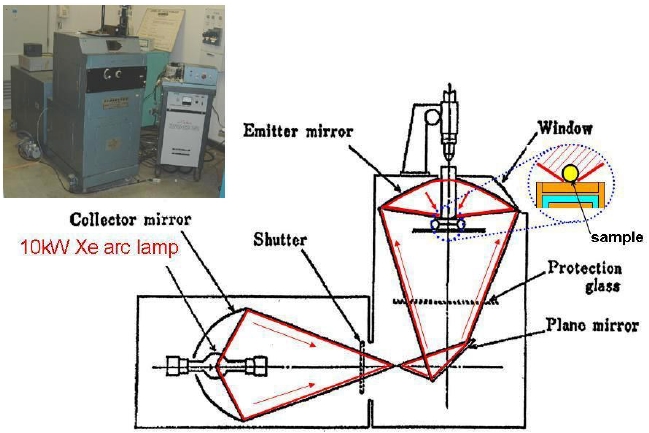
Figure 1 shows the schematic diagram of the melt synthesis reactor. This furnace is designed to heat small-size samples up to very high temperature, like above 2,000℃, to melt in clean conditions. In the melt synthesis, the mixture of raw materials is melted in a short period of time (from 1 to 60s) by a strong light radiation in an arc imaging furnace. A spherical molten sample with multiple cations was mixed homogeneously at around 2,000℃, and directly solidified on a Cu hearth, after closing the shutter. The cooling rate was estimated to be more than 100 ℃/s. Excitation and emission spectra in the UV range were measured for the crushed powder sample, using a spectrofluorometer (FP- 6500/6600; Jasco Inc.).
2.2 Synthesis by fluidized bed reactor
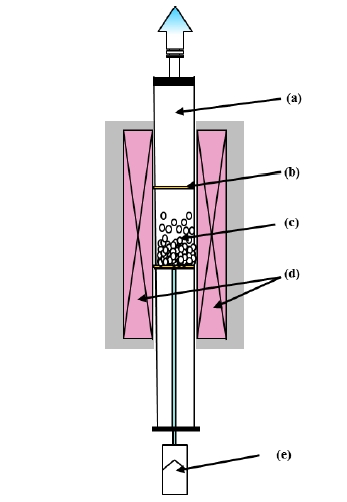
Figure 2 shows the schematic diagram of the fluidized bed reactor. An alumina tube was placed vertically as a reactor, and starting material powders were placed on a porous Al2O3 or SiC filter at the center of the alumina tube. The synthetic conditions of temperature, heating time, reaction gas ratio N2 and NH3, and gas flow rate were optimized, based on experimental results. Phase purity of the products was characterized by powder Xray diffraction (XRD) analysis. Optical properties of the nonaggregated powder products were examined by UV-Vis diffuse reflectance spectra and spectrofluorometer.
2.3 Vapor-solid hybrid synthesis
Large size phosphor powder samples were synthesized, using
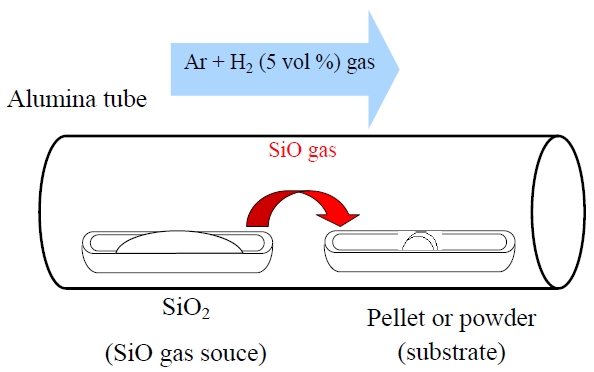
a new vapor phase technique. The vaporization of SiO was performed at 1,400-1,600℃ from the SiO2 source (or SiO powder) in a reductive atmosphere. The SiO gas reacted with raw material powders or pellets. The reference sample was synthesized by the solid-state reaction method. Figure 3 shows a schematic diagram of the vapor phase reactor.
Conventional Y3Al5O12:Ce3+ (YAG: Ce) phosphor displays bright yellow emission by blue LED excitation. The conventional synthesis technique of LED phosphor material is a solid state reaction, from the mixture of oxide or carbonate starting materials. The YAG: Ce phosphor synthesized by the high temperature solid state reaction shows agglomerate and irregular shape particles, with inhomogeneous distribution of Ce3+ ions, because of poor reactivity of the starting materials. The synthesis of such complex oxide phosphor is not easy by conventional solid-state reaction techniques. The reaction rates among oxides are very slow by solid-state diffusion, because of high melting points of the raw materials, Al2O3 (melting point 2,054 ± 4℃) and Y2O3 (2,433 ± 3℃).
As shown in Fig. 4, the phosphors synthesized by the “melt
technique” show a superior luminescence property, because of high crystallinity. Figure 5 shows a SEM image of the as-prepared spherical molten sample (phosphor ball). The melt process results in a well-grown YAG:Ce phosphor with a lens-like morphology. The lens-like phosphor ball was placed in front of an emitting blue LED, to see which blue light was transmitted, and whether luminescent conversion occurred. This was done by directly applying a melt phosphor lens consisting of rare earth phosphors to the LED, to obtain a color balance.
3.2 Synthesis by fluidized bed reactor
Compared to the batch type reactor system, the fluidized bed reactor system has some advantages: (i) the whole part of solid
reactants can be kept at a steady temperature, and (ii) heat can be transferred very fast to substances, because each reactant is constantly fluidized. In particular, compositionally uniformed nitride and oxynitride phosphor powders were synthesized from metal oxide powders, using a fluidized bed reactor in a NH3 flow.
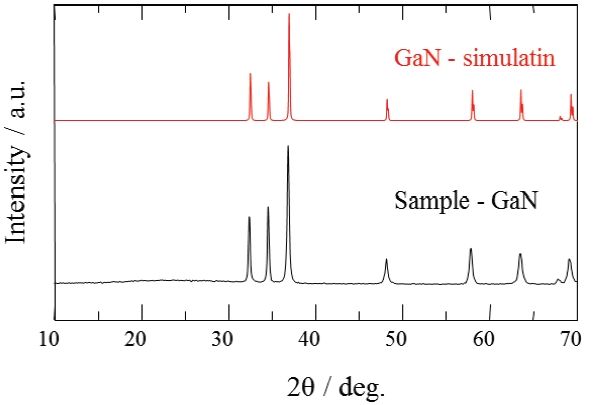
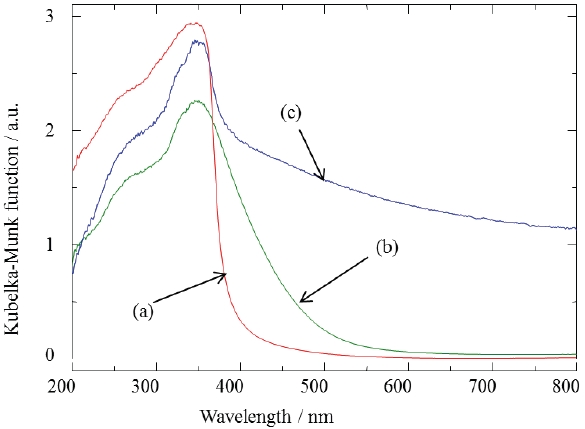
Figure 6 shows XRD patterns of the GaN samples synthesized in pure NH3 flow. A single phase of GaN phosphor powder was obtained by nitriding in the fluidized bed reactor. Figure 7 shows UV-Vis diffuse reflectance spectra of the GaN samples. The color of the powder synthesized by the fluidized bed technique was pure white. On the other hand, the color of the powder synthesized by a conventional solid state reaction was yellow or dark gray. The color of nitride compounds prepared from the cor-
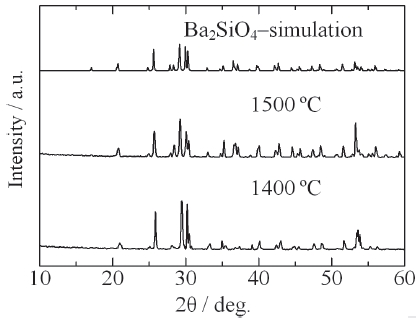
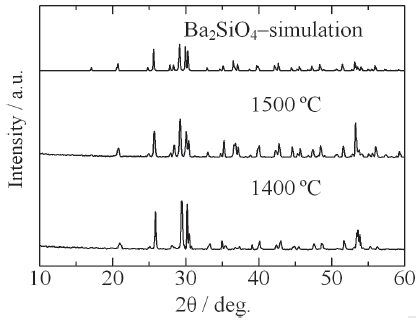
[Fig. 9.] XRD patterns of Ba2SiO4:Eu2+ phosphor synthesized by the new vapor-solid hybrid technique.
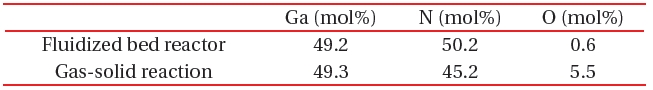
[Table 1.] A comparison of composition between the fluidized bed reaction and gas-solid reaction.
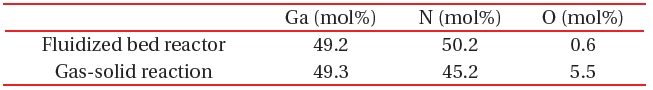
A comparison of composition between the fluidized bed reaction and gas-solid reaction.
responding oxides depends on the conversion ratio of nitrogen and oxygen in nitriding reactions. The GaN powders synthesized at high temperatures also showed a tendency to be black in color.
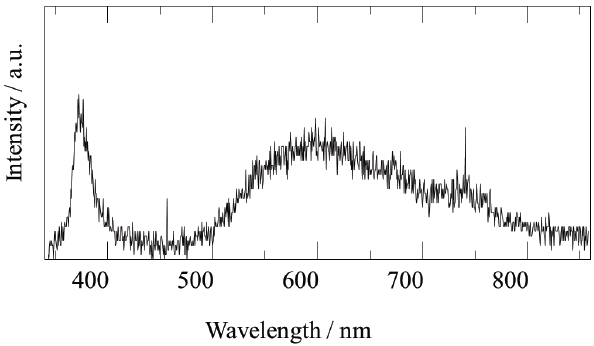
A fluidized bed has uniform reactivity in the whole reactor, because each reactant is constantly fluidized. Therefore, we synthesized compositionally uniformed powders of GaN, using a fluidized bed reactor with a gas-solid reaction system, as shown in Table 1. High quality GaN powder exhibited the band-edge emission at about 370 nm, without defect emission peak around the green-yellow regions, as shown in Fig. 8.
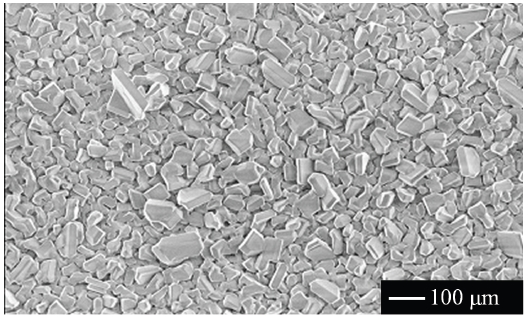
3.3 Vapor-solid hybrid synthesis
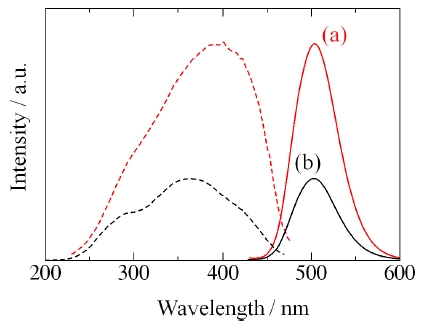
Figure 9 shows the XRD patterns of Ba2SiO4:Eu2+ phosphor synthesized by the new vapor-solid hybrid technique. Green emitting phosphor powders were deposited on the Ba-Eu-O system pellet at 1,400-1,500℃ for 12 h. Figure 10 shows a SEM image of the Ba2SiO4:Eu2+ crystals grown on the surface of the pellet at 1,500℃ for 12 h. Well-grown powders of 10-40 micrometer size were mainly observed, because of relatively slow nucleation. Heating of the SiO2 source (or SiO powder) in a strong reductive atmosphere (Ar-H2) generated gaseous SiO, which reacted with Ba- and Eu-oxides. Figure 11 shows the excitation and emission spectra of the Ba2SiO4:Eu2+ phosphor at room temperature. The Ba2SiO4:Eu2+ phosphor synthesized by the new vapor phase technique has a relatively strong absorption band in the long wavelength side. The emission intensity of the phosphor synthesized
by the new vapor phase technique at 1,500℃ is about 2.6 times higher than that of a conventional solid state reaction sample. This is due to high crystallinity, and relatively few defects of the vapor phase processed sample.
Well-crystallized and grown LED phosphors were synthesized by the new synthesis techniques, of “melt synthesis”, “fluidized bed synthesis” and “vapor-solid hybrid synthesis”. The LED phosphor powders were obtained in a quite short reaction time for these reactions, which is a new, useful and powerful method for synthesis of LED phosphors.