Biomedical materials play a key role in modern medicine. Material scientists have tried various techniques and different components to fabricate biomedical materials according to specific clinical requirements. To achieve successful regeneration of damaged tissues, artificial substrates that can mimic certain features of the natural extracellular matrix have shown their advantages. Fibrous structures have been identified as being more favorable for cell adhesion, proliferation, and differentiation, owing to their similarity to biological structures [1-3]. Bone-tissue regeneration membranes have recently attracted extensive interest because a considerable number of teeth can be preserved from prospective extraction due to periodontal diseases through bone-tissue regeneration therapy [4-7]. Bone tissue is composed of collagen nanofibers and hydroxyapatite (particularly carbonated hydroxyapatite) nanocrystals. Thus, the electrospun collagen/hydroxyapatite (HA) [3,8] and gelatin/HA [4] composite fibers have been reported on intensively as being effective in repairing bone defects and mimicking the composition and structure of natural bone tissue. It has been found that electrospun composite fibrous scaffolds, including poly(L-lactic acid) (PLLA)/collagen (or gelatin) [9,10], polycaprolactone (PCL)/collagen (or gelatin) [11,12], and poly(lactide-co-glycolide) (PLGA)/collagen [13,14], can maintain their original patterns with little change in fiber morphology and interfiber distances after being crosslinked. The reason for this is the presence of a hydrophobic polyester component that prevents fiber swelling and conglutination.
HA, a major inorganic component of bone, has been used for biomedical implant applications and bone regeneration due to its bioactive, biodegradable, and osteoconductive properties [15- 17]. Being similar to the mineral component of natural bone, this substance has showed good osteoconductivity and bone bonding ability [18]. However, whether used in block or granular forms, pure HA cannot degrade in the human body. To combine the osteoconductivity of calcium phosphates and the biodegradability of polymers, polymer/ceramic composites have been developed for bone tissue engineering either by direct mixing or by biomimetic approaches [19,20]; especially, HA ceramics were also improved by mixing with tough polymers. The nano size of the inorganic component (mainly bone-like apatite) in natural bone is considered to be important for the mechanical properties of the bone [21]. Recent studies in this field have also suggested that better osteoconductivity would be achieved if synthetic HA could resemble bone minerals more in composition, size, and morphology [22,23]. In addition, nanosized HA may have other special properties due to its small size and huge specific surface area. Webster et al. [24] have shown significant increase in protein adsorption and osteoblast cell adhesion on the nanosized ceramic materials compared to traditional micron-sized ceramic materials.
PLLA is now being used in biomedical fields due to its good biodegradability; however, this material requires some modifications in terms of biocompatibility and medical properties for its extension to certain applications. Thus many researchers have studied the modification of the biocompatibility of polymer composites with inorganic materials for tissue engineering [25,26]; among such materials, composites of HA have been used clinically in various forms, such as spheres, films, or scaffolds [27- 29]; these composites have been shown to improve the bone-cell response
The electrospinning technique was invented in 1934 and can be used to produce polymer fibers with nanometer to micrometer diameter size [32]. Recently, this technique has been introduced to the field of tissue engineering [33]. Numerous materials have been spun into nanofibers by static electric forces; these new materials possess a microscopic three-dimensional porous structure and are shown macroscopically to be membranes. It has been reported that such membranes show good biocompatibility [34-36].
Carbon nanofibers (CNFs) show good mechanical properties, chemical stability, high aspect ratio, and surface properties that allow easy functionalization with more biocompatible hydrophilic groups; these materials have attracted considerable attention for both fundamental scientific understanding and their potential biomedical applications [37,38]. In order to have sufficient bonding between CNFs and juxtaposed bone, and to minimize motion-induced damage to surrounding tissue in situ, a combination of CNFs with biomaterials is a very effective approach. It is generally believed that β-tricalcium phosphate (β-TCP) has a chemical composition close to that of bone and can be gradually degraded in tissue and interstitial fluids [39,40].
Cell culture data has proved that coelectrospun polyester/col-lagen (or gelatin) composite fibrous scaffolds show potential in tissue reconstitution and regeneration [13,41]. Various kinds of simulated body fluids (SBF) have been used by different authors to deposit Ca-P minerals on polymeric scaffolds in various forms, including solid-walled [42] and fibrous scaffolds [43]. Periodontal ligament stem cells are stem cells found near the periodontal ligaments of the teeth. They are involved in adult regeneration of the periodontal ligament, alveolar bone, and cementum. The cells are known to express STRO-1 and CD146 proteins. Osteoblast cells are the large cells responsible for the synthesis and mineralization of bone during both initial bone formation and later bone remodeling. Osteoblasts form a closely packed sheet on the surface of the bone, from which cellular processes extend through the developing bone. They arise from the differentiation of osteogenic cells in the periosteum, the tissue that covers the outer surface of the bone, and in the endosteum of the marrow cavity. This cell differentiation requires a regular supply of blood, without which cartilage-forming chondroblasts, rather than osteoblasts, are formed. The osteoblasts produce many cell products, including the enzymes alkaline phosphatase, and so on.
In this paper, we review the processing methods of various nanofiber/nanoparticle reinforced PLLA composite membranes and cell (periodontal ligament cell [PDLC], gingival epithelial cell [GEC], osteoblast-like MG 63) culturing on membranes for bone tissue regeneration
2.1. Fabrication of PLLA, PLLA/HA, PLLA/ MWNTs/HA membrane
Fig. 1 shows a schematic diagram of the PLLA/multiwalled carbon nanotubes (MWNTs)/HA membrane fabrication process.
MWNTs were first modified by anodic oxidation; then, MWNTs/ HA nanoparticles were in situ synthesized by a wet method with Ca(NO3)2?4H2O and (NH4)2HPO4 (Ca/P = 1.67) by using an ultrasonic vibrator. The prepared nanoparticles were repeatedly washed with 1, 4-dioxane to remove water; particles were then dispersed again in 1, 4-dioxane to form a suspension. Dichloromethane and PLLA particles were added to the suspension until a weight ratio of MWNTs/HA to PLLA of 1:9 was achieved. The suspension was kept in a 50℃ oven for 12 h in order to obtain a mixed solution. Before electrospinning, ultrasonic stirring of the solution was maintained for 1 h. The solution was electrospun continuously with a programmable syringe pump (Top 5300, Japan) from a 20 mL syringe with a steel needle (inner dia. 0.5 mm) at a rate of 0.7 mL/h. Voltage (15 kV) was applied to the tip of the needle by use of a high-voltage supply when the fluid jet was ejected.
2.2. Fabrication of PLLA/TFE membrane
Fig. 2 shows schematic views of the PLLA/trifluoroethanol (TFE) nanofibrous membrane fabricated by electrospinning. To obtain randomly arranged PLLA nanofibrous membranes, a metal plate (20 × 25 cm2) was used as a collector at a distance of 18 cm from the tip of the needle. To obtain parallel PLLA nanofibrous membranes, a cylindrical drum, which rotated at a surface linear rate of 12 m/s, was used as a collector at a distance of 18 cm from the tip of the needle. To achieve hyperparallel PLLA nanofibrous membranes, the membranes were cut into 6 cm lengths along the fiber orientation; cuttings were 5 cm in width. The membranes were fixed on a stretching set, as shown in Fig. 2c, and drawn by using a 300 g weight at 100℃ for 5 min along the fiber axial direction. Before cell culturing, all PLLA nanofibrous membranes were exposed to a vacuum oven at room temperature for 2 weeks to remove residual solvent.
2.3. Fabrication of PLLA/gelatin membrane
PLLA and gelatin (1:1 in weight ratio) were weighed and dissolved in 2,2, 2-TFE (99%). After being stirred overnight at
room temperature, a viscous solution with a concentration of 10 wt% was obtained. The solution was loaded into a syringe fixed with a stainless steel needle (inner dia. 0.37 mm) and electrospun. The desired flow rate was set at 0.4 mL/h. The applied voltage was kept at 12 kV. A flat aluminum plate, 20 cm away from the needle tip, was used as the collector.
2.4. Fabrication of β-TCP/CNFs membrane
Fig. 3 shows a schematic diagram of the β-TCP/CNFs membrane fabrication process. Triethyl phosphate (TEP, 2.28 mL) was mixed with 10 mL distilled water, with stirring at 353 K for 48 h, to obtain a hydrolyzed TEP solution. Calcium nitrate tetrahydrate (CN, 4.72 g) was dissolved into the hydrolyzed TEP solution, and solution was stirred at room temperature for 120 h to generate a calcium-phosphorous complex. Then, 1 mL of the prepared solution was added to 10 mL N, N-demethylformamide (DMF, >99, 5%) containing 10 wt% polyacrylonitrile (PAN), and stirred for 6 h at room temperature to obtain a homogeneous solution. Electrospinning and fabrication of the β-TCP/CNFs membrane followed the method in the previous report [35].
2.5. Characterization of membranes
The electrospun PLLA, PLLA/HA, and PLLA/MWNTs/HA membranes were gold ?coated using sputter coating in order to observe the surface morphology and average diameter by scanning electron microscope (SEM). An energy-dispersive X-ray spectroscopy (EDX) analysis was carried out to confirm the extent of P and Ca elements and the distribution of HA particles in the membranes. A Raman spectrum analysis was conducted to confirm the existence of MWNTs in the PLLA/MWNTs/HA membranes. Membranes were cut into rectangles (20 × 20 × 0.05 mm) for an in vitro degradation test. The structural properties of membranes, such as surface area, pore volume, and average pore size, were investigated from nitrogen adsorption-desorption isotherms using a surface area analyzer. Mechanical properties were tested with an Instron (Model 1121) at 20 mm/min crosshead speed with 10 mm gauge length at room temperature.
2.6. PDLCs culture on membranes
PDLCs were obtained from healthy teeth extracted for orthodontic reasons. Informed consent was obtained from the patients before the extractions. The midmost of the periodontal ligament attached
to the root surface was removed with a curette, cut into small pieces, and cultured in a tissue culture medium. An α-modification of Eagle’s medium (α-MEM) containing 10% fetal bovine serum and antibiotics was used to culture the cells. After reaching 80% confluence, the cells were passaged with 0.25% trypsin/0.02% ethylene diaminotetraacetic acid (EDTA). The cells between the third and the fifth passages were used in the following studies. PDLCs were harvested with 0.25% trypsin/0/02% EDTA and transferred to an osteogenic differentiation medium. After 7 days culturing, PDLCs were transfected using a recombinant adenovirus containing green fluorescent protein at a ratio of 1:20 for 2 h. A flow cytometer was used to determine the ratio of transfection. Membranes were cut into a round shape, 10 mm in diameter, to fully cover the bottoms of wells of tissue culture plates; membranes were then fixed. PDLCs were seeded into different kinds of membranes and tissue culture polystyrene (TCPS: control group) at a density of 5000 cells/well and cultured in an osteogenic differentiation medium. After 1, 3, 5, and 7 days culturing, cells were detached with 0.25% trypsin/0/02% EDTA at 37℃ for 10 min, repeatedly pipetted, centrifuged, and resuspended. The number of suspended cells was counted using a hemocytometer under an inverted fluorescence microscope. The number of viable cells was also determined with MTT assay, which has previously been described [46].
2.7. Human GECs culture on membranes
GECs were obtained from the gingival tissue of systematic healthy individuals removed during periodontal surgery. The explants were treated with 6 mg/mL of Dispase in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline at 4℃ to separate the epithelium from the underlying fibrous connective tissue. The epithelium was then removed and incubated at 37℃ in 0.25% trypsin/0.02% EDTA for 10 min and repeatedly pipetted to prepare a single-cell suspension. Further experiments were followed by PDLC culture.
2.8. Osteoblast cell culture on membranes
PLLA and PLLA/HA hybrid membranes were added into Dulbecco’s modified eagle medium (DMEM) and incubated at 37℃ for 24 h. Then, membranes were removed and the extracts were stored at 4℃ for further use. Osteoblast cells (MG-63) were cultured in DMEM with or without the extracts for 24, 72, and 120 h. The number of viable cells was measured by MTT assay. After sterilization, the hybrid membranes were transferred into 24 well plates and held by a metal ring. Osteoblast cells in 1 mL DMEM were plated on the membranes. After 1 h, each membrane was topped up with enough culture medium and incubated for 2 more days. Then, the constructs were washed with phosphate buffer solution to remove nonadherent cells, were fixed with 4% glutaraldehyde for 1 h at room temperature, were dehydrated through a series of graded alcohol, and were air dried overnight. These dry constructs were gold-coated and cell adhesion on membranes was observed using SEM.
Fig. 4 shows the characteristics of PLLA, PLLA/HA, PLLA/ MWNTs/HA membranes [35]. The diameters of the three kinds
of electrospun fibers were about 1 μm, and three dimensional porous structures were obtained. The incorporation of HA or MWNTs/HA nanoparticles resulted in fibers that were more irregular in diameter and more beaded in morphology than those derived using pure PLLA fibers. EDX analysis shows the presence of elemental P and Ca in the membranes. The Raman spectra confirmed the presence of MWNTs in the membranes. A crystalline carbon apex and an amorphous carbon apex, which are the two unique apexes of MWNTs, were also found in the PLLA/MWNTs/HA membrane.
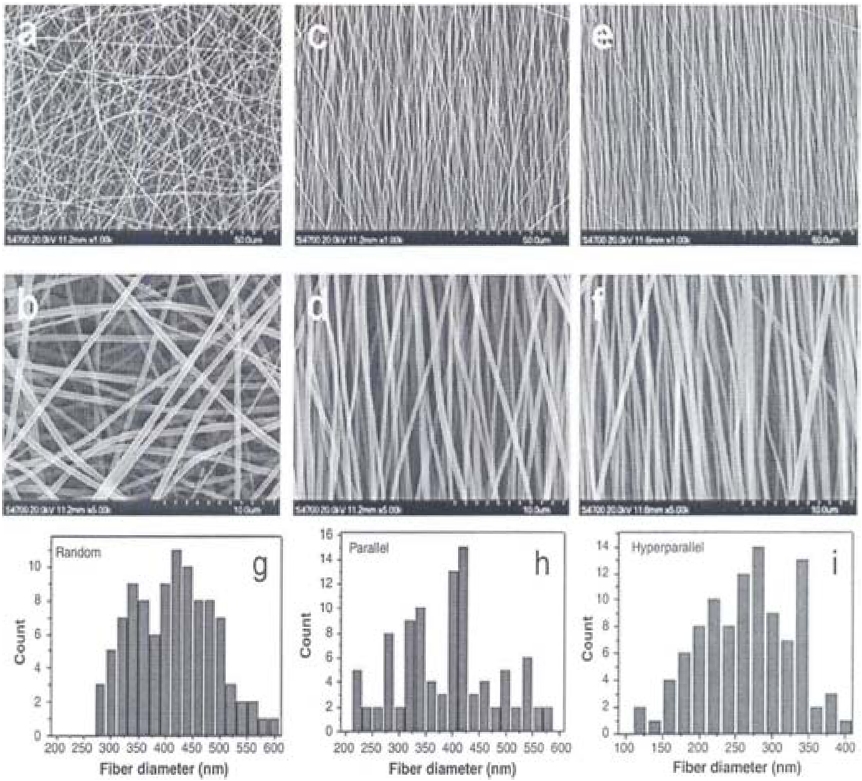
Fig. 5 shows SEM images of electrospun membranes with different fiber arrangements. The random PLLA nanofibers showed isotropic fiber alignments with average fiber diameter of 450 nm. The parallel and hyperparallel fibers exhibited anisotropic alignments with average diameters of 325 and 275 nm. Especially, the hyperparallel group showed a higher order of fiber arrangement than that of the parallel group due to the hot stretching of the membrane.
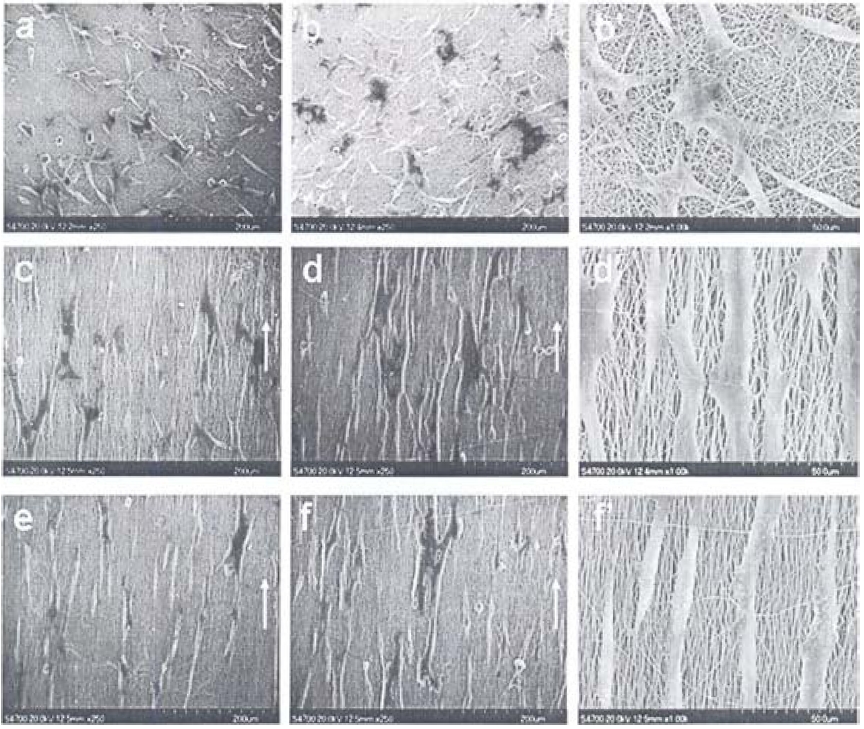
Fig. 6 provides SEM images of as-electrospun (a) PLLA, (b) PLLA/gelatin, and (c) gelatin fibers, having smooth surface and 1 μm diameter [36]. After crosslinking, severe morphological deformation and conglutination had occurred on the gelatin fibers, causing damage to the porous structure, while the PLLA/ gelatin composite fibers maintained their original structure. The three kinds of fibrous scaffolds showed different levels of hydrophilicity. Hydrophobic PLLA fibrous scaffolds showed surface water contact angles around 123° during the whole monitoring time, while the contact angle of pure gelatin fibrous scaffolds leveled off at 42°. However, a water drop was detected soaking
into the PLLA/gelatin fibrous scaffolds within 20 s and a final contact angle of 0° resulted.
Fig. 7 shows SEM, transmission electron microscope (TEM), and macroscopic images of β-TCP/CNFs [40]. β-TCP/CNFs emerge as an ultra-thin black web with a thickness ~30 μm; these structures exhibit a millimeter-length scale fibrous morphology with a partial alignment along the rolling direction
and an average diameter of 120 nm. Some nanoparticles were exposed on the surface of the CNTs (Fig. 6B). TEM affiliated energy dispersive spectroscopy (EDS) indicated that the content of β-TCP/CNF nanocrystalline structures in the CNFs was about 15 wt%. An HR-TEM image of a typical CNF distinctly shows that it emerges in a disordered lattice structure with a low degree of crystallization.
Fig. 8 shows X-ray diffraction (XRD) patterns of the CNFs and β-TCP/CNFs carbonized at 1373 K in N2 [40]. The broad and weak diffraction peaks at 2
nanoparticles from the calcium-phosphorous complex through high temperature carbonization.
Fig. 9 shows mass loss and pH change of three kinds of membranes during in vitro degradation [35]. The weights of all membranes were found to continuously decrease due to the degradation of fibers. However, the incorporation of HA or MWNTs/HA nanoparticles slowed down the degradation of PLLA because the dissolving alkaline HA particles acted as a physical barrier that was able to block the entry of water. The pH of the solution decreased continuously for the PLLA membrane. On the other hand, the pH of the PLLA/HA and PLLA/MWNTs/HA membranes decreased in the beginning step, but, 4 weeks later, it slowly increased due to HA’s releasing of OH- after degradation, which neutralized the acid resulting from PLLA degradation [47].
Fig. 10 shows tensile stress-strain curves of PLLA nanofibrous scaffolds with random, parallel, and hyperparallel fiber arrangement in the scaffolds. As the orientation increased, the PLLA nanofibrous scaffold, especially the hot-stretched portion, showed a significantly increased tensile strength and modulus.
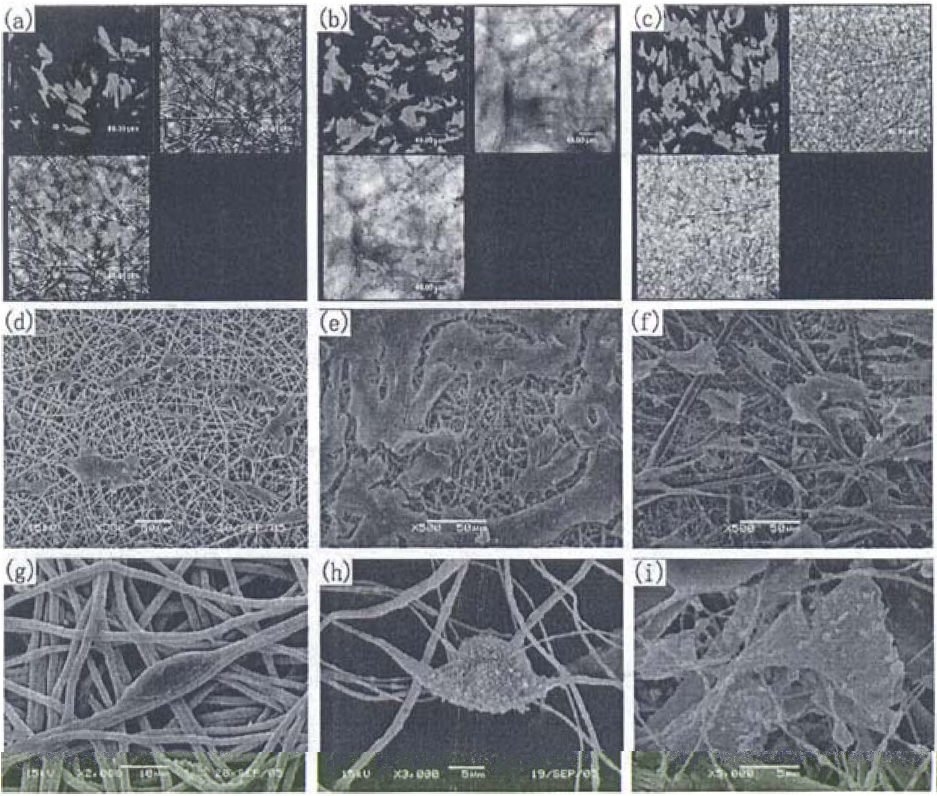
Fig. 11 shows the PDLC culture on three kinds of membranes [35]. The density of the PDLCs on the PLLA/MWNTs/HA membrane was the highest among those of the three membranes. The cells spread over the membrane fibers, linked with fibers by cytoplasmic extension. PDLCs were more actively extended on the PLLA/HA membrane than on the other two membranes during the same culturing time. However, the PLLA/MWNTs/HA membrane was the most suitable for human PDLC adhesion and proliferation. Wutticharoenmongkol et al. [48] reported that hydroxyapatite nanoparticles introduced nanofibrous scaffolds that showed excellent tissue regeneration. Chen et al. [49] reported that the fiber diameter in the scaffolds played an important role in tissue regeneration.
Fig. 12 shows the effect of the membranes on the adhesion and proliferation of PDLCs and GECs, which processes were examined by cell counting and MTT assay [35]. The PDLC number was similar at 1 day for the three test and control groups, while the most active proliferation was observed on the PLLA/ MWNTs/HA membrane, showing a level almost 3 times that of the initial seeding cells and 30% larger than that of the PLLA/ HA membrane or that of the control group for the 7 day culture. On the other hand, fewer human GECs were found to attach to the PLLA/MWNTs/HA membrane, resulting in the inhibition of GECs.
Fig. 13 shows histological examinations of osteoblast cells/ membranes implanted into immunodeficient mice [35]. It was observed that residual electrospun fibers could be clearly identi-
fied; no obvious inflammation was found in the implant areas. Bonelike tissues were formed with round or irregular shapes and were stained into homogeneous pink by hematoxylin/eosin; osteoblast-like cells were well arranged around the bonelike tissues. Calcium deposits were confirmed in the newly-formed bonelike tissue by alizarin red staining. It is notable that abundant blood vessels were grown in the newly-formed tissues. Osteocalcin, which was stained in brown, was detected in the cytoplasm and outside the cells. The results indicated that the PLLA/
MWNTs/HA membrane was of good biocompatibility in vivo during the 4 week period, and human PDLCs seemed to func-
tion well on the membrane. The three dimensional porous structures of the implanted membranes may be helpful to guide the ingrowth of blood vessels, which ingrowth plays an important role in tissue regeneration. Commercially available guided tissue regeneration (GTR) membranes such as Bio-Gide (collagen type I and III) and Guidor (PLLA, Guidor AB) employ a twolayer design [5,50]. A three-layer nanocarbonated hydroapatite/ collagen/PLGA composite membrane was introduced for GTR and showed an enhanced protein content of osteoblastic cells cultured on the membrane [6,7].
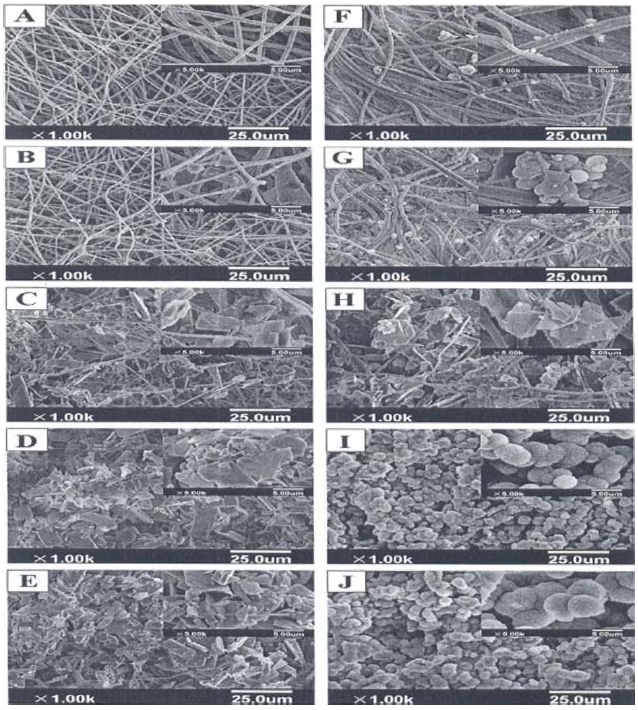
Fig. 14 provides SEM images of the PLLA and PLLA/gelatin membranes, which were immersed in 5 x SBF for 3-24 h [36]. A large amount of apatite minerals were deposited on the scaffolds. PLLA/gelatin composite fibrous scaffolds kept their original porous structure without obvious fiber swelling, although there was a presence of a hydrophilic gelatin component [51]. In the first few hours (3-6 h), inorganic depositions could hardly be seen on the fiber surface. As the biomineralization proceeded, sheet-like mineralites appeared on the scaffolds. The fibers were totally covered by the apatite layer after they were soaked in 5 x SBF for 18-24 h. Different from the remaining sheet-like deposition on the PLLA scaffolds, the sheet-like deposition on the PLLA/gelatin composite fibers transformed into spherical mineralites at 24 h. The sheet-like and spherical mineralites coexisted at all times on the composite scaffolds, as shown in Figs. 12f-j.
Fig. 15 shows CLSM images of the PDLCs cultured on (a) CNF and (b) β-TCP/CNF membranes for 24 h. PDLCs can be adhered favorably on both membranes with proliferating preference along the aligned longitudinal direction of the nanofibers. Figs. 13c-f shows the proliferation of PDLCs and the deposition of the extra-cellular matrix on the membranes. For the 1 day culture, PDLCs were well adhered along the fiber direction and were interlinked with nanofibers by cytoplasmic extensions. The oriented proliferations of PDLCs confirm the good physiochemical compatibilities of the CNFs and β-TCP/CNFs; this result coincided well with the aforementioned confocal laser scanning microscopy (CLSM). After 7 days of culturing, the cells obviously proliferated on the membrane surfaces with increasing coverage areas, and PDLCs were more actively extended on the β-TCP/CNFs membrane. Ogose et al. [39] also reported the effect of β-TCP as bone substitution material after excision of bone tumors.
Fig. 16 shows the proliferation rates of MG-63 cells on PLLA nanofibrous scaffolds. Cell density increased from the 1 day to the 7 day culturing in all groups, although there was no statistical difference among the random and parallel scaffolds and the TCP group. The hyperparallel group exhibited a significant lower cell density than that of other groups.
Fig. 17 shows the morphologies of the MG-63 cells cultured on the PLLA nanofibrous scaffolds with random, parallel, and hyperparallel alignment. For all scaffolds, MG-63 cells adhered and grew well, and the cells appeared to interact and associate with the surrounding fibers. The cells cultured on the random scaffold showed polygonal forms with no obvious orientation, while for both the parallel and the hypoparallel aligned scaffolds the cells showed polarized forms with orientation along the fiber direction. Notably, it could be seen that more filopodia like extensions and filament like structures extended from the MG-63 cells on the random PLLA fibers than on the parallel and hyperparallel fibers (Figs. 8b’, d’, f’). Ko et al. [52] reported on the biological behavior of MG-63 cells on hydroxyapatite surfaces.
In this paper, we have reviewed the fabrication and cell culturing of various CNF/nanoparticle reinforced PLLA related membranes. Electrospinning is a recommendable technique to obtain favorable membranes. Human cells such as PDLCs, osteoblast-like cells that can be effectively used for bone-tissue regeneration, were well adhered and proliferated on the membranes. On the other hand, GECs, which are bad for humans, were successfully inhibited on the membranes.
![Schematic diagram of PLLA/MWNTs/HA membrane fabrication process [37]. PLLA: poly-L-lactic acid, MWNTs: multiwalled carbon nanotubes, HA: hydroxyapatite.](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f001.jpg)
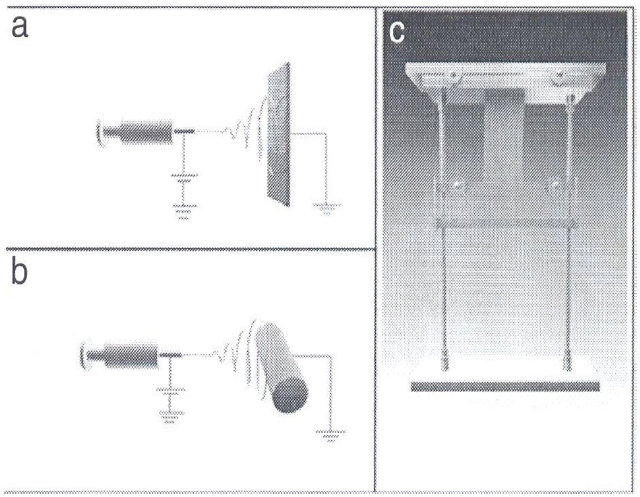
![Schematic diagram of poly-L-lactic acid/carbon nanotubes membrane fabrication process [42].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f003.jpg)
![Characterization of three kinds of membranes: (a-c) represent scanning electron microscope images of PLLA, PLLA/HA, and PLLA/MWNTs/HA membrane, (d) represents energy-dispersive X-ray spectroscopy mapping of PLLA/HA and PLLA/MWNTs/HA membranes for elemental Ca and P, (e) represents Raman spectra of MWNTs (upper), PLLA/HA (middle), and PLLA/MWNTs/HA membrane (lower), respectively [37]. PLLA: poly-L-lactic acid, MWNTs: multiwalled carbon nanotubes, HA: hydroxyapatite.](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f004.jpg)
![Scanning electron microscope images of as-electrospun (A) poly- L-lactic acid (PLLA), (B) PLLA/gelatin, (C) gelatin fiber, and crosslinked (D) and (E). (F-H) Water drop images on each membrane [38].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f006.jpg)
![(A) Scanning electron microscope, (B) transmission electron microscope (TEM), and (C) macroscopic images of β-tricalcium phosphate (β-TCP)/carbon nanofibers (CNFs). HR-TEM images and selected area electron diffraction patterns (inset) of (D) carbon nanotubes and (E) β-TCP nanoparticles in the β-TCP/CNFs membrane [42].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f007.jpg)
![Characteristics of three kinds of membranes during in vitro degradation: (a) and (b) represent changes of mass and pH [37].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f009.jpg)
![X-ray diffraction patterns of (A) carbon nanofibers (CNFs) and (B) β-tricalcium phosphate/CNFs carbonized at 1373 K in N2 for 2 h [42].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f008.jpg)
![Typical tensile stress-strain curves of poly-L-lactic acid nanofibrous scaffolds with different fiber orientations [42].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f010.jpg)
![Effect of membranes on the adhesion and proliferation of periodontal ligament cells and gingival epithelial cells: (a) and (c) represent cell number, (b) and (d) represent the MTT assay [37]. PLLA: poly-L-lactic acid, MWNTs: multiwalled carbon nanotubes, HA: hydroxyapatite, TCPs: tricalcium phosphates.](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f012.jpg)
![Histologic examinations of cell/membranes implanted into immunodeficient mice: (a-c) show newly formed bonelike tissues with round or irregular shapes (white arrow); osteoblast-like cells were well arranged around bonelike tissues. Abundant blood vessels were found in the implanted area. In (c), alizarin red staining confirmed calcium deposits in newly-formed bonelike tissues; in (d), osteocalcin, which was stained in brown, was detected in the cytoplasms outside the cells [37].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f013.jpg)
![Confocal laser scanning microscopy images of periodontal ligament cells (PDLCs) cultured on (A) carbon nanofibers (CNFs) and (B) β-tricalcium phosphate (β-TCP)/CNFs membrane (red: actin, blue: cell nucleus), stained by TRITC-phalloidin and Hoechst 33342; scanning electron microscope images of PDLCs cultured on (C) CNFs and (D) β-TCP/ CNFs membrane for 1 day, (E) and (F) for 7 days. The arrows indicate the longitudinal direction of the nanofibers [42].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f015.jpg)
![MG-63 cell proliferation on poly-L-lactic acid nanofibrous scaffolds with different fiber orientations after 1,3,5, and 7 days culture [47].](http://oak.go.kr/repository/journal/11282/HGTSB6_2012_v13n3_139_f016.jpg)