Electrochemical capacitors or supercapacitors have attracted increased interest due to their high power density compared to that of conventional capacitors [1-3]. Electrochemical capacitors have been developed to provide power pulses for a wide range of application areas including transportation, consumer electronics, medical electronics, and military devices [4]. According to the energy storage mechanism, two kinds of electrochemical capacitors have been examined: 1) electric double layer capacitors (EDLC) using carbon having a very high surface area as the electrode material [5]; 2) conducting polymers [6] or metal oxide based pseudo-capacitors [7].
In pseudo-capacitors, charge is stored by the electrical charge transport in redox reaction on the electroactive species. These species in pseudo-capacitors formed metal oxides and conducting polymers on the electrode surface, accelerating the redox reactions [8,9].
Polypyrrole (PPy) is one of the most important conducting polymers; it is used in a wide range of applications owing to its relatively facile processability, mechanical flexibility, low cost, electrical conductivity, and thermal and chemical stability [10].
Carbon nanotubes (CNTs), as EDLC electrode materials, have attracted much attention because of their high electrical conductivity, unique entangled network, and dominant porous character [11]. Nowadays, significant interest has been generated in research that mixes CNTs with various inorganic and organic substances by physical and chemical approaches to prepare multifunctional composites. A variety of CNT composites have been prepared and investigated [12,13]. Recently, extensive efforts have been made to prepare functional PPy- CNT composites that exhibit enhanced electrical, thermal or mechanical properties relative to PPy or to CNTs alone [14].
Much research on supercapacitors is aimed at increasing both the power and the energy densi-ty as well as lowering fabrication costs while using environmentally friendly materials. The development of advanced composite materials based on the use of metal oxide-carbon nanotubes for supercapacitor electrodes has been studied in the hope that these materials can provide improved capacitive behaviors due to their enhanced stability, high conductivity, and pseudo-capacitance [15,16].
In this work, a novel PPy-coated Fe3O4/ multi-walled CNT (MWNT) nanoparticle composite (Fe-MWNT/PPy) has been prepared by the coprecipitation of Fe3+ and Fe2+ and the in situ polymerization of pyrrole. The characterization and electrochemical performance of Fe-MWNTs/Ppy are performed to determine its potential use as a supercapacitor electrode.
MWNTs produced via chemical vapor deposition method were purchased from Nano Solution Co. (Korea, degree of purity: ≥95 wt%, diameter; 10-25 nm, length: 10-50 μm). Ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), pyrrole, ammonium persulfate (APS), and ammonium hydroxide aqueous solution (NH4OH, 25 wt%) were obtained from Aldrich.
The pristine MWNTs were purified by impregnating them with 5 M HNO3 for 5 h at room temperature. Purified MWNTs was washed using distilled water until pH 7.0 was obtained. After vacuum filtration, the sample was dried for 24 h at 120℃ in a vacuum oven.
Fe-MWNTs were prepared by suspending 1.0 g of purified MWNTs in 200 mL of distilled water containing 1.0 g FeCl2·4H2O and 2.7 g FeCl3·4H6H2O at 50℃ under an N2 atmosphere. Then, 0.5 mL of NH4OH solution was slowly added to the above solution under continuous stirring to promote complete growth of the nanoparticle crystals. Subsequently, the composites were centrifugally separated from the solution, washed with distilled water until a pH 7.0 was reached, and dried at 100℃ for 12 h in vacuum. The chemical reaction of the Fe3O4 precipitation is as follows [17].
2.3. Preparation of Fe-MWNTs/PPy
To prepare Fe-MWNT/PPy, the obtained Fe-MWNTs were dispersed in 150 mL of 0.1 M HCl solution. Subsequently, 1 mL of pyrrole was added. After the solution was stirred mechanically for 10 min, it was mixed with an aqueous solution of APS. The polymerization of pyrrole was allowed to proceed for 5 h at approximately 0-5℃ . Then, the final product was washed with copious amounts of water and absolute ethanol and was dried under vacuum at 80℃ for 24 h.
The structures of the MWNTs and Fe-MWNTs were characterized using X-ray diffraction (XRD, BRUKER D2 PHASER) with CuKα radiation. The XRD patterns were obtained in 2 θ ranges between 20? and 80? at a scanning rate of 5? /min.
The surface characteristics of the MWNTs, Fe-MWNTs, and Fe-MWNTs/PPy were determined using X-ray photoelectron spectroscopy (XPS, VG Scientific Co., ESCALAB MK- II) with an Al Kα (hν = 1486.6 eV) X-ray source.
For the electrochemical investigation, cyclic voltammetry (CV) and galvanostatic charge-discharge tests were performed using an electrochemical analyzer (Iviumstat, Ivium Technologies) to evaluate the electrochemical performance in the potential range between -0.9 V and 0 V in the 1 M Na2SO3 electrolyte solution.
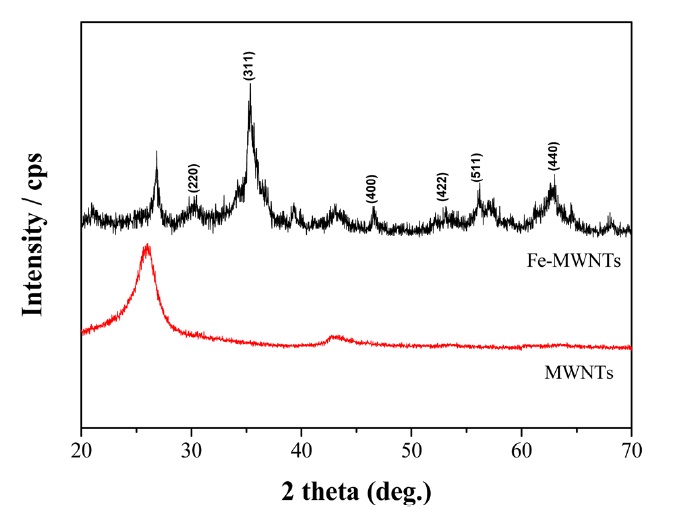
Fig. 1 shows XRD patterns of the MWNTs and Fe-MWNTs. Diffraction peaks assigned to the MWNTs at 2θ = 26.5? and 43? can be clearly seen in the XRD curves of the MWNTs and Fe- MWNTs, indicating that the MWNT structure was not destroyed after the successive deposition of Fe3O4. The XRD patterns exhibit typical characteristic peaks of Fe3O4 at 2θ = 30.2? , 35.4? , 46.6? , 53.7? , 57.3? , and 62.8? , which is in agreement with the standard values (JCPDS card no. 75-0033). The grain size of the Fe3O4 particles was calculated from the major diffraction peak (311) using the Debye-Scherrer equation [18].
where Lc is the particle size (nm), K is the Scherrer constant (= 0.9), λ is the X-ray wavelength (CuKα = 0.154 nm), θ is the angle at the peak maximum, and β1/2 is the width (radians) of the peak at half the height. According to equation, the deposited Fe3O4 particles have an average grain size of 14 nm.
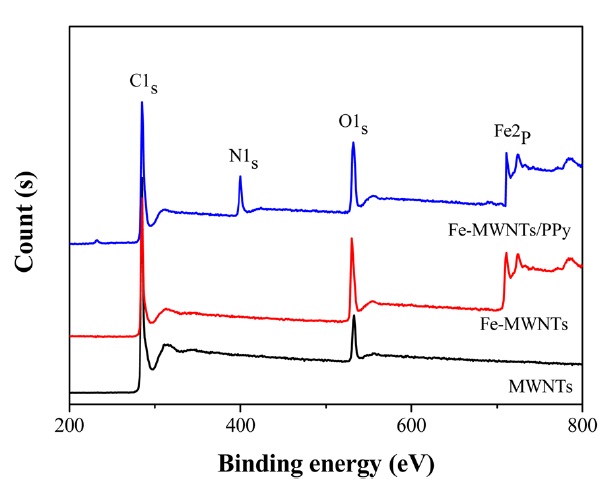
The MWNTs, Fe-MWNTs, and Fe-MWNTs/PPy were also characterized using XPS analysis. Fig. 2 shows the wide scan spectrum, in which the photoelectron lines at binding energies of about 285, 533, 400, and 711 eV are attributed to C1s, O1s, N1s, and Fe2p, respectively. The peaks at 285 eV correspond
with the sp2 hybridized carbon of the MWNTs [19]. The N1s spectrum of Fe-MWNTs/PPy shows its peak at a binding energy of about 400 eV, which is characteristic of amine-like or neutral pyrrolium nitrogens (-NH- structure) [20]. In the case of Fe2p, the core-level spectra of the Fe-MWNTs and Fe- MWNTs/PPy show very similar peaks at a binding energy of about 711 eV. It can be suggested that there is no significant chemical reaction between PPy and Fe-MWNTs, and that Fe-MWNTs are used as a template for the polymerization of conducting PPy.
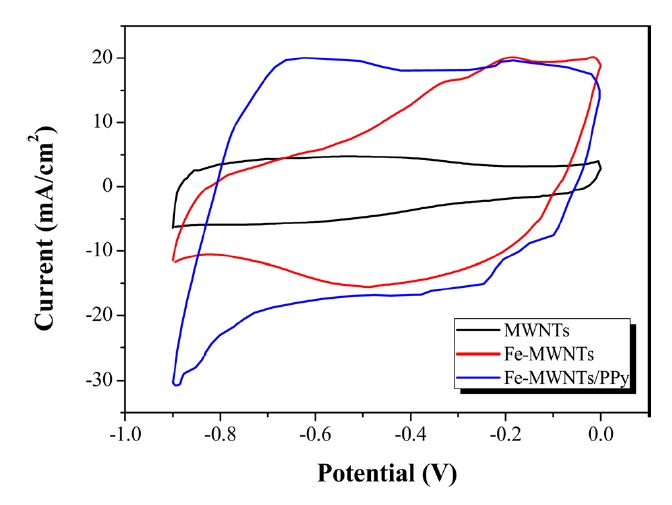
The electrochemical performance of MWNTs, Fe-MWNTs, and Fe-MWNTs/PPy was measured using CV and charge-discharge curves in 1 M Na2SO3 electrolyte solution. Fig. 3 presents the CV curves of each electrode between a potential from -0.9 to 0 V at a scan rate of 50 mV/s. It can be seen that the MWNT electrodes have symmetrical, rectangular-shaped voltammograms, which is a characteristic feature of EDLCs. However, anodic peaks and cathodic peaks were observed in the CV for the Fe-MWNTs and Fe- MWNTs/PPy electrodes, which is attributed to the redox reactions of Fe3O4. The possible mechanism is as follows [21]:
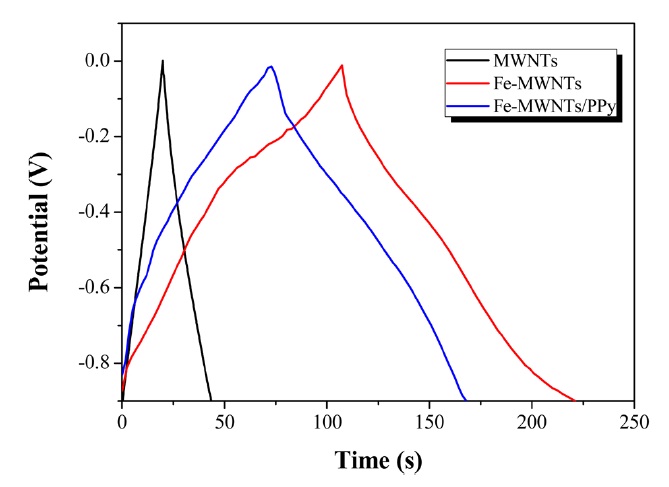
The galvanostatic charge-discharge curves of MWNT, Fe- MWNT, and Fe-MWNTs/PPy electrodes were investigated at 0.5 A/g; the corresponding results are shown in Fig. 4. It can be seen that the curve of the MWNT electrode is very symmetrical, indicating that the MWNT electrode has perfect EDLC behavior. However, the shapes of the charge-discharge behavior of the Fe- MWNT and Fe-MWNTs/PPy electrode did not show the characteristics of a pure electrochemical double-layer capacitor, which is in agreement with the result obtained from CV in Fig. 3.
The specific capacitance can be measured by the galvanostatic charge-discharge behavior, according to the following equation [22]:
where I is the current of charge-discharge, t the discharge time, ΔV the potential window, and m the mass load of active materials. The calculated specific capacitances of the MWNTs, Fe-MWNTs, and Fe-MWNTs/PPy are 20, 60, and 73 F/g, respectively. It is obvious that the specific capacitance of the Fe- MWNTs/PPy is improved by the coating of PPy, which enhances the electric conductivity, lowers the resistance, and facilitates the charge-transfer of the composites.
In this work, Fe-MWNTs/PPy was successfully prepared by the coprecipitation of Fe3+ and Fe2+ and the in situ polymerization of pyrrole. From the CV results, the prepared Fe-MWNT and Fe-MWNTs/PPy electrodes showed pseudo-capacitive characteristics in terms of their electrochemical reversibility and reactivity. Charge-discharge measurement of the prepared electrodes at 0.5 A/g yielded specific capacitances of 20, 60, and 73 F/g for MWNTs, Fe-MWNTs, and Fe-MWNTs/PPy, respectively. Among these electrodes, the Fe-MWNTs/PPy electrode exhibited excellent capacitive performance and had a high specific capacitance from -0.9 to 0 V in 1 M Na2SO3 electrolyte due to pseudo-capacitance stemming from the redox reaction of Fe3O4 and the high electric conductivity and increased charge transfer between Fe-MWNTs and PPy.