Nanoporous carbons have strong potential for application in various fields, including energy and environmental fields in particular [1,2]. Extensive research has been carried out to develop efficient storage media of supercritical gases as a clean energy and a realistic alternative to the compression and liquefaction storage techniques of gases. Nanoporous carbons are considered a promising candidate for supercritical gas storage [3,4]. As the interaction of a supercritical gas molecule with the pore wall of nanoporous carbon is not strong enough, highly ultramicroporous (pore width < 0.7 nm) carbons are predicted to be efficient adsorption sites for supercritical gases [4]. Supercapacitors, another promising candidate for application of nanoporous carbons, have attracted much attention as energy storage devices for electric vehicles and hybrid electric vehicles [5]. For obtaining high-performance supercapacitors, several parameters of nanoporous carbons are recognized as key factors in terms of their application as electrode materials: specific surface area, pore size, conductivity, and surface chemical state. The selection of an optimal electrolyte has also been demonstrated to be an important factor for maximizing the performance of supercapacitors [6,7]. In particular, the relationship between the pore size of the nanoporous carbons and electrolyte ion size is an important parameter in enhancing the specific capacitance of supercapacitors [8].
[Table 1.] Activation conditions of PVDC-derived nanoporous carbons
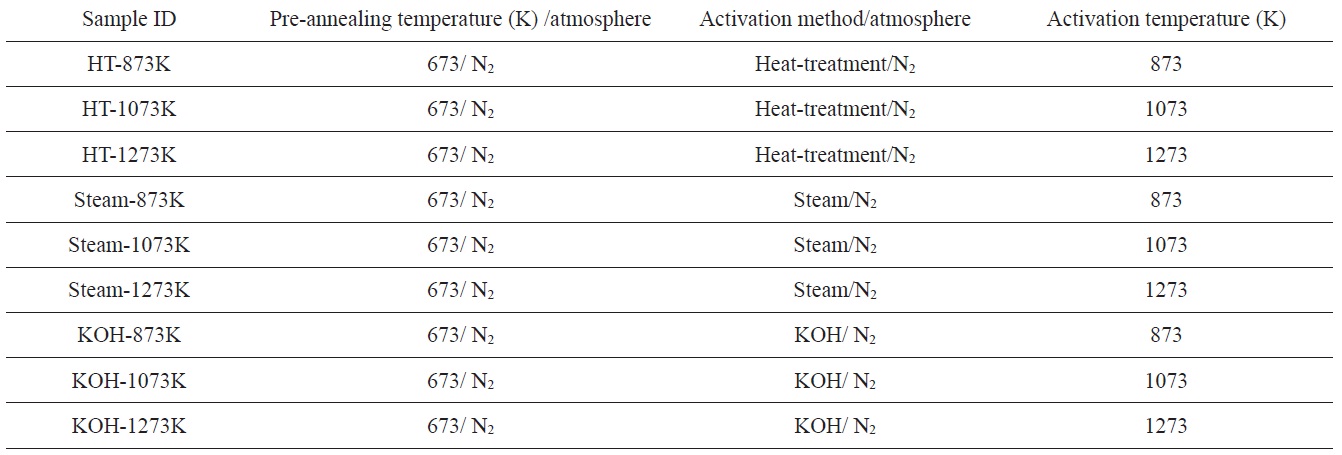
Activation conditions of PVDC-derived nanoporous carbons
Pore size and pore geometry of nanoporous carbons are important governing factors in the adsorption behaviors of various guest molecules [9-11]. The pores of nanoporous materials are classified according to the pore width w, which is the shortest distance in three-dimensional geometry, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). The micropores have two subgroups of ultramicropores and supermicropores (0.7 nm < pore width < 2 nm). The maximum size of ultramicropores corresponds to the bilayer thickness of the adsorbed N2 molecules. Although the importance of ultramicropores has been emphasized in molecular sieving and supercritical gas adsorption, an accurate evaluation of the ultramicroporosity is very difficult due to the blocking near the pore entrance. Recently, many researchers have often used the term “nanopores,” which is not recommended by the IUPAC. Nanopores can be defined as having size less than 5 nm, which is convenient owing to the inherent adsorption characteristics according to recent progress in adsorption science and technology.
Poly(vinylidene chloride) (PVDC) has been proposed as a promising precursor material for obtaining nanoporous carbons [8,12-22]. It is well known that PVDC-derived nanoporous carbons could be prepared by simple heat-treatment without an additional activation process [8,12-20]. The PVDC-derived nanoporous carbons prepared by heat-treatment alone provide an ultramicroporous structure. In this study, various activation methods for PVDC were performed in order to control the porosity of the PVDC. The effects of the activation conditions on the porosity changes were investigated by the N2 adsorption technique at 77 K.
Homogeneous PVDC (Asahi Kasei Co.) with a crystallite size of 26.7 nm was annealed at 673 K in a N2 atmosphere for 1 h before the activation process. Activation of PVDC was performed by three methods. First, for the heat-treatment method, the preannealed PVDC samples were further heat-treated at 873, 1073, and 1273 K in a N2 atmosphere for 1 h, respectively. Second, for the steam activation method, the pre-annealed samples were further heat-treated at the same temperature in a steam atmosphere for 1 h. N2 was used as a carrier gas. Third, for the KOH activation method, mixtures of pre-annealed PVDC samples and potassium hydroxide (KOH) were further heat-treated at the same temperature in a N2 atmosphere for 1 h. The weight ratio of KOH to PVDC was 2:1. The N2 flow rates for all experiments were 100 mL min-1. The sample names and treatment conditions are summarized in Table 1.
The electrical conductivity of PVDC-derived porous carbons was measured with a powder resistivity measurement system. The powder samples were compressed in a cylinder cavity with a diameter of 21 mm under controlled pressure in a range of 3.6~125.4 MPa at room temperature. The pore structures were determined by N2 adsorption at 77 K using volumetric equipment (Micromeritics ASAP 2010) after preevacuation at 423 K for 2 h, while maintaining the base pressure at 10-4 Pa. Pore structure parameters were obtained by the subtracting pore effect (SPE) and Dubinin-Radushkevich (DR) methods. The SPE method was performed by using high-resolution αs-plots, which are constructed using the standard adsorption data for nonporous carbon black [23,24].
3.1. Electrical conductivity of PVDC-derived nanoporous carbons
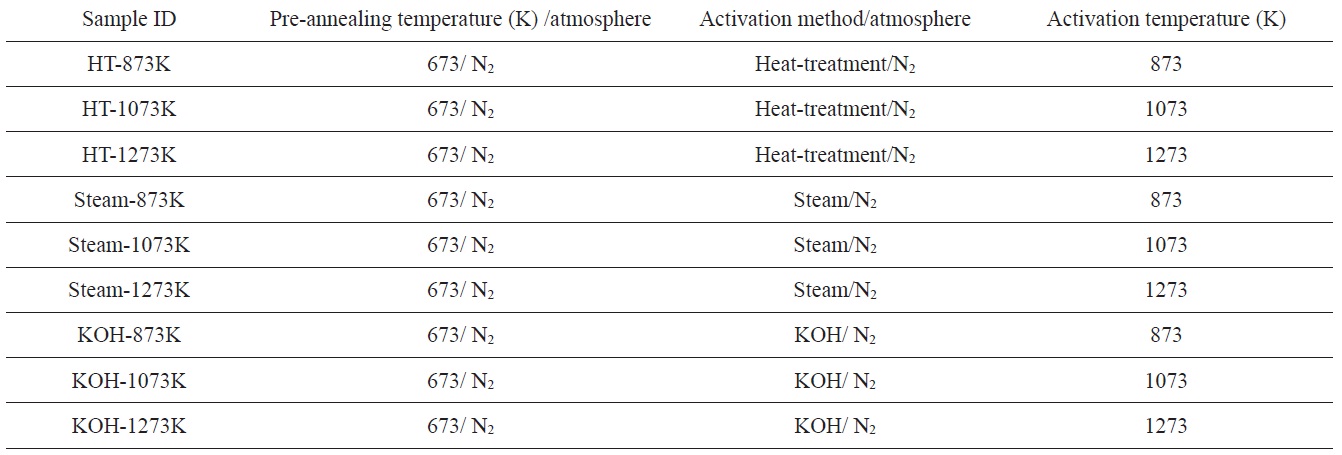
Fig. 1 shows the powder resistivity of PVDC-derived nanoporous carbons as a function of applied pressure. The electrical conductivities of all samples increased with an increase in applied pressure, suggesting that the contact area between adjacent particles of the nanoporous carbon increases with increasing applied pressure. The relationship between the electrical conductivity of pressed particles and activation temperatures is also
shown in Figs. 1b-d. The electrical conductivity of all samples increases with the activation temperature. Therefore, the electrical conductivity strongly depends on the activation temperature of the PVDC. In general, the electrical conductivity of porous carbons decreases with increasing porosity, which is associated with the formation of isolated conducting pathways. However, thermal decomposition of PVDC eliminates non-carbon atoms and develops a more layered carbon structure by carbonization behavior, resulting in enhanced electrical conductivity. In our results, as heat-treatment at higher temperature accelerates the carbonization as well as the development of a porous structure of the PVDC, the electrical conductivity of the samples should be enhanced. Therefore, to obtain nanoporous carbons with better electrical conductivity, it is important to control the activation temperature for PVDC.
3.2. Pore structure of PVDC-derived nanoporous carbons
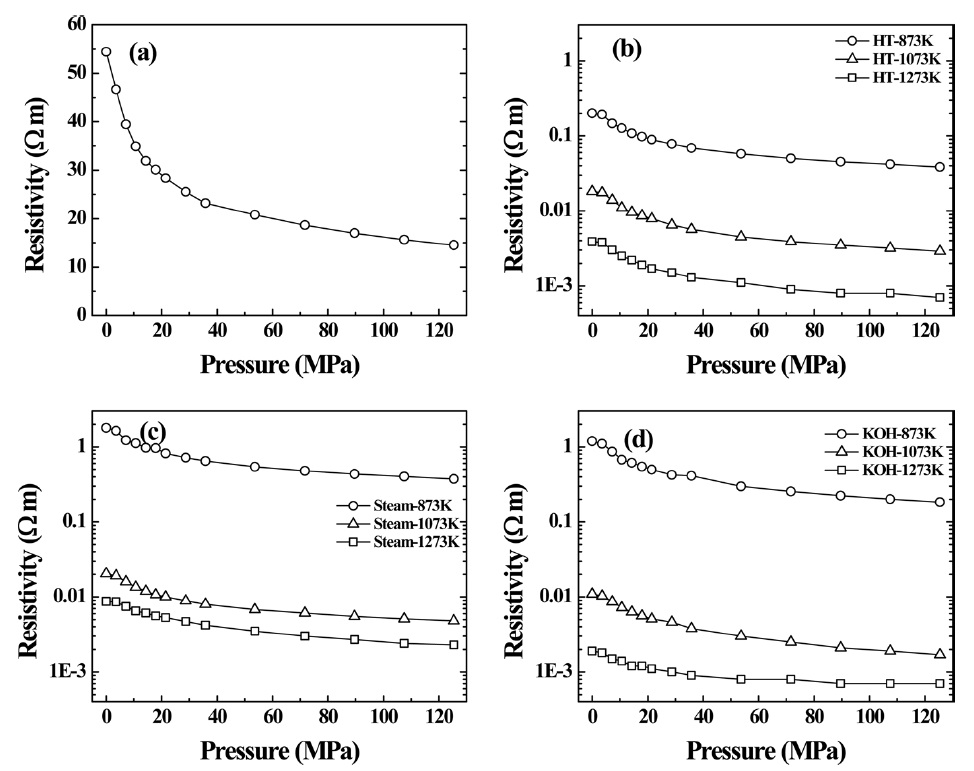
Fig. 2a shows N2 adsorption isotherms (77 K) of PVDCderived nanoporous carbons heat-treated under an inert atmosphere. All adsorption isotherms are of Type I, suggesting the presence of uniform microporosity. The saturated amounts of N2 adsorption at relative pressure (
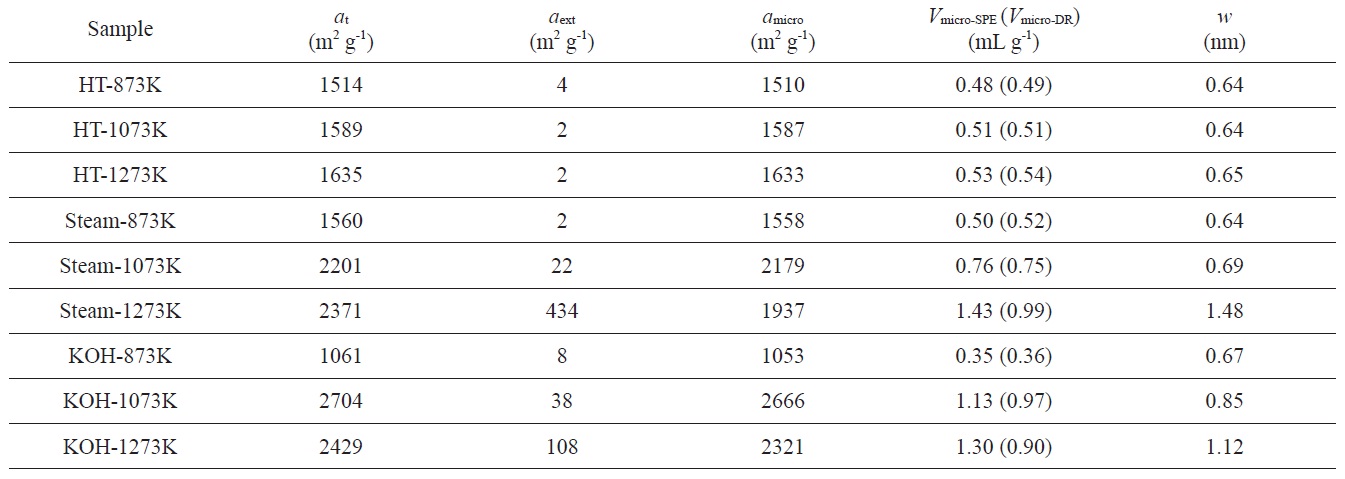
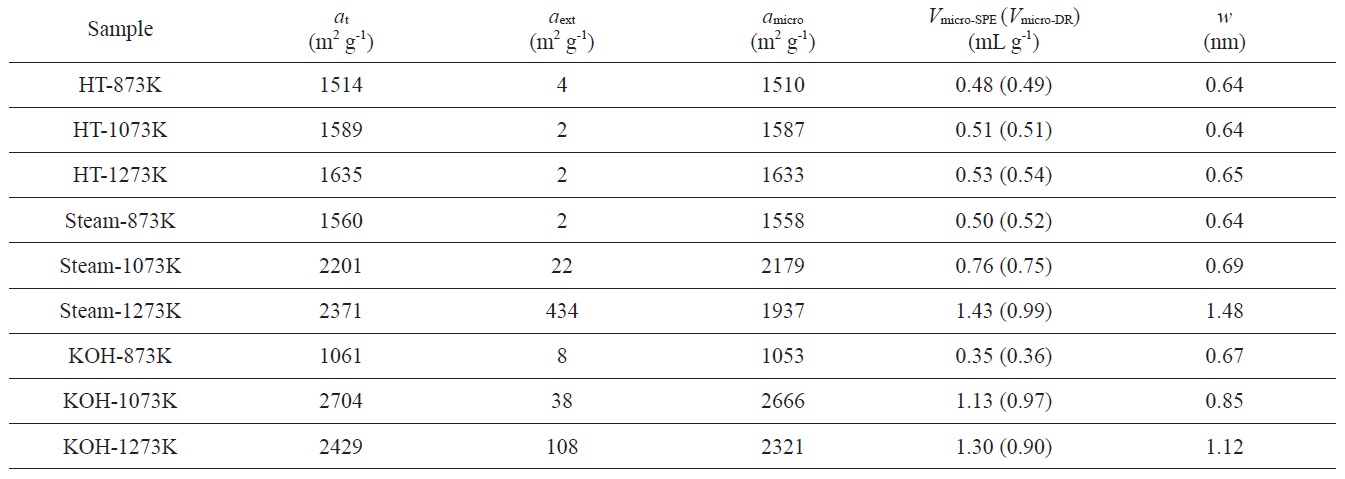
Pore structure parameters of PVDC-derived nanoporous carbons determined by SPE method. Micropore volumes (Vmicro-DR) were determined by the DR method
tramicroporous carbons. The formation of an ultramicroporous structure of the PVDC-derived nanoporous carbon heat-treated in an inert atmosphere is thought to be closely related to complete release of hydrogen and chlorine atoms during thermal decomposition of the PVDC at high temperature [25].
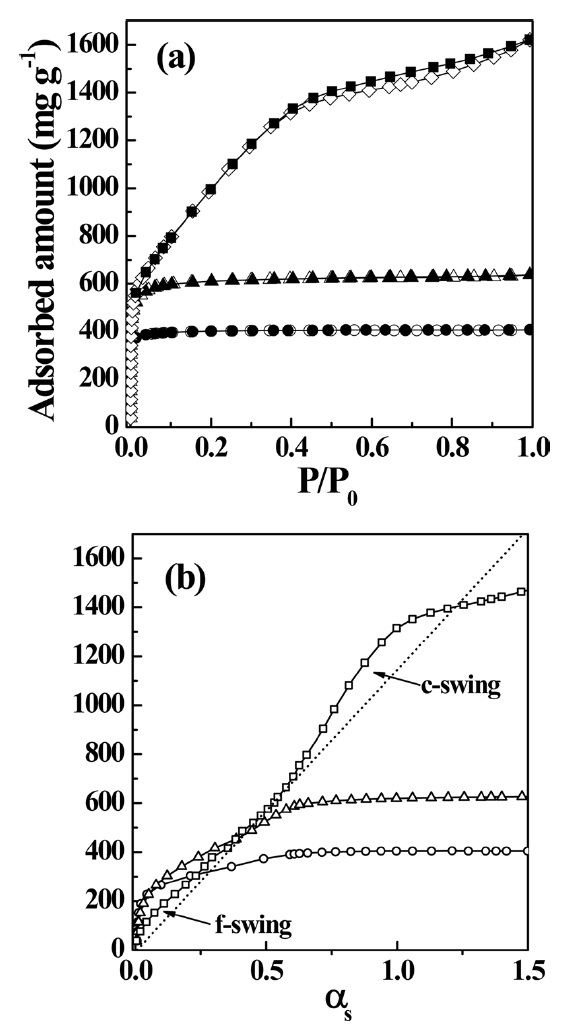
Fig. 3a shows N2 adsorption isotherms of PVDC-derived nanoporous carbons activated under steam. The N2 adsorption isotherms of activated samples at 673 and 873 K are also Type I, which is due to the presence of uniform microporosity. The saturated amounts of N2 adsorption at
treated at 1273 K dramatically decreases to about 0.7, which is associated with the presence of supermicropores, in good agreement with the average pore width determined by the SPE method. Therefore, steam activation at higher temperature has advantages for creating supermicropores. This should be attributed to pore formation by the elimination of hydrogen and chlorine atoms and to successive pore widening due to the attack of more oxidative steam at higher temperature.
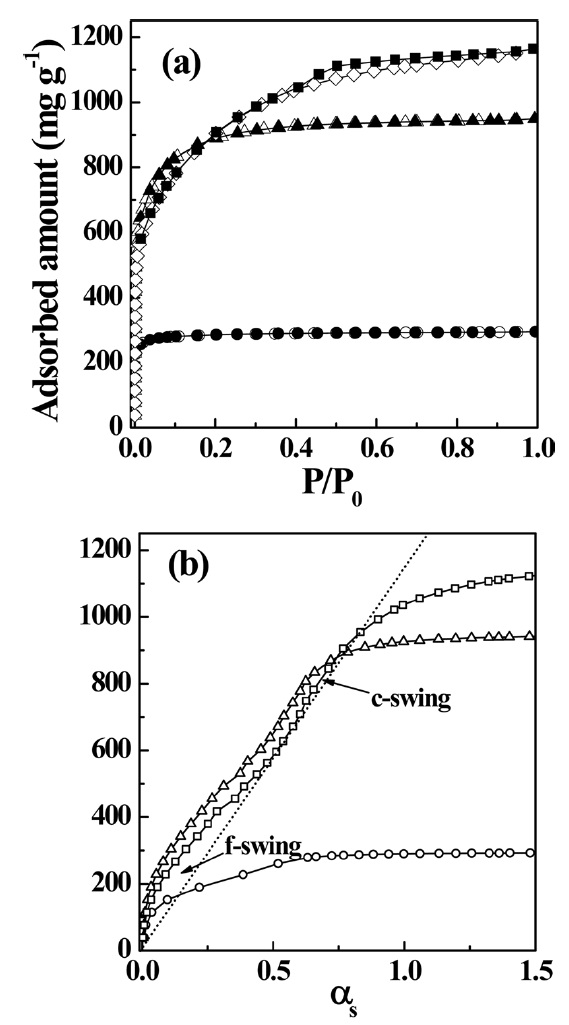
Fig. 4a presents N2 adsorption isotherms of PVDC-derived nanoporous carbons chemically activated with KOH. The N2 adsorption isotherms of all samples are defined as type I. However, the isotherm of the sample treated at 1273 K shows a gradual adsorption uptake until
αs-plot of the sample treated at 1273 K shows a f-swing and c-swing in the αs region of 0.5 to 1.0, suggesting the presence of both ultramicropores and wider micropores, as shown in Fig. 4b, which reflects more heterogeneous microporosity. The micropore volume increases with activation temperature. In contrast, the specific and micropore surface areas of the sample treated at 1273 K slightly decrease compared to those of the sample treated at 1073 K. The chemical activation at higher temperature also results in widened average micropore width. The average micropore width is linearly proportional to the activation temperature, indicating a strong dependence on the activation temperature of the pore structure. As shown in Table 2, the ratio of
Nanoporosity control of PVDC-derived porous carbons was successively achieved by various activation methods. Heat-treatment in an inert atmosphere for PVDC provided an ultramicroporous structure. On the contrary, steam activation at 1273 K provided a supermicroporous structure with average micropore width of 1.48 nm. With elevated KOH activation temperature, the average micropore width was gradually enlarged from 0.67 to 1.12 nm. Therefore, the average pore widths of KOH-activated samples were strongly governed by the activation temperature. Consequently, PVDC-derived nanoporous carbons with controllable pore size can find useful applications as electrode materials for energy storage devices.