Peragallo (1892) regarded the genera
Sundstrom (1986) suggested that only those species with valves bearing an external process, otaria, claspers, and copulae perforated by loculate areolae should be included in the genus
More recently, the family Rhizosoleniaceae included
In Korea, the genera
Field samples were collected in Korean coastal waters from September 2008 to February 2010 (Table 1). Phyto-plankton was collected using a 20 μm mesh-sized net by vertical towing. Samples were immediately fixed in neu-tralized formalin (final concentration 4%), glutaraldehyde (final concentration 2%), and Lugol’s solution. Organic material in the samples was removed using the methods of Hasle and Fryxell (1970) and Simonsen (1974). The ma-terials were examined under a light microscope (Axios-kop 40; Carl Zeiss, Jena, Germany), photographed with a MRc5 camera (Carl Zeiss) and a scanning electron micro-scope (JSM-5600LV; Jeol, Tokyo, Japan). Sizes of cells were measured using image calculation software (AxioVision AC v. 4.5; Carl Zeiss).
Terminology was from that recommended in the first report of the working Committee on Diatom Terminol-ogy (Anonymous 1975) from the third Symposium on Recent and Fossil Marine Diatoms, Kiel. Other terminol-ogy follows Ross et al. (1979), Sundstrom (1986), Round et al. (1990), Hernandez-Becerril (1995), and Hasle and Syvertsen (1996).
We identified 6 rhizosolenid genera, including
Class Bacillariophyceae Haeckel 1878
Order Centrales Hustedt 1930
Suborder Rhizosoleniineae Simonsen 1979
Family Rhizosoleniaceae De Toni 1890
Genus Proboscia Sundstrom 1986
Proboscia alata (Brightwell) Sundstrom 1986
Proboscia indica (H. Peragallo) Hernandez-Becerril 1995
Genus Neocalyptrella (Norman) Hernandez-Becerril & Meave 1996
Neocalyptrella robusta Hernandez-Becerril & Meave 1996
Genus Pseudosolenia Sundstrom 1986
Pseudosolenia calcar-avis (Schultze) Sundstrom 1986
Genus Guinardia H. Peragallo 1892
Guinardia delicatula (Cleve) Hasle 1995
Guinardia flaccida (Castracane) H. Peragallo 1892
Guinardia striata (Stolterforth) Hasle 1995
Genus Dactyliosolen Castracane 1886
Dactyliosolen fragilissimus (Bergon) Hasle 1995
Dactyliosolen phuketensis (Sundstrom) Hasle 1995
>
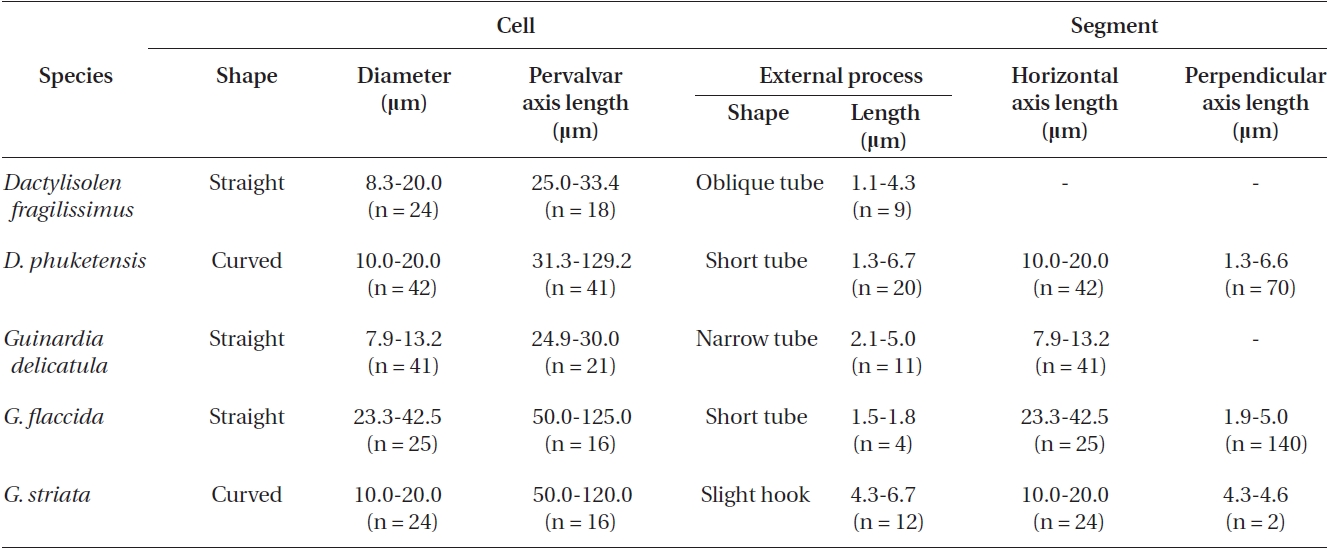
Proboscia alata (Brightwell) Sundstrom 1986 (
Brightwell 1858, p. 95, Pl. 5, Fig. 8; Peragallo 1892, p. 115, Pl. 18, Fig. 11-20; Hustedt 1920, Pl. 317; Hustedt 1930, p. 600, Fig. 345; Cupp 1943, p. 90, Fig. 52A & B; Oku-no 1952, p. 353, Pl. 2, Fig. 5 & 6; Okuno 1960, p. 310, Pl. 1, Fig. 1; Hendey 1964, p. 146, Pl. 2, Fig. 2; Drebes 1974, p. 57, Fig. 39c & d; Navarro 1981, p. 430, Fig. 33 & 34 as R. alata; Sundstrom 1986, p. 99, Fig. 258-266; Jordan et al. 1991, p. 65, Fig. 1-9; Takahashi et al. 1994, p. 413, Fig. 2-7; Hernandez-Becerril 1995, p. 252, Fig. 2-4; Hasle and Syvertsen 1996, p. 159, Pl. 30; Sunesen and Sar 2007, p. 639, Fig. 82-88 & 98.
Synonyms.
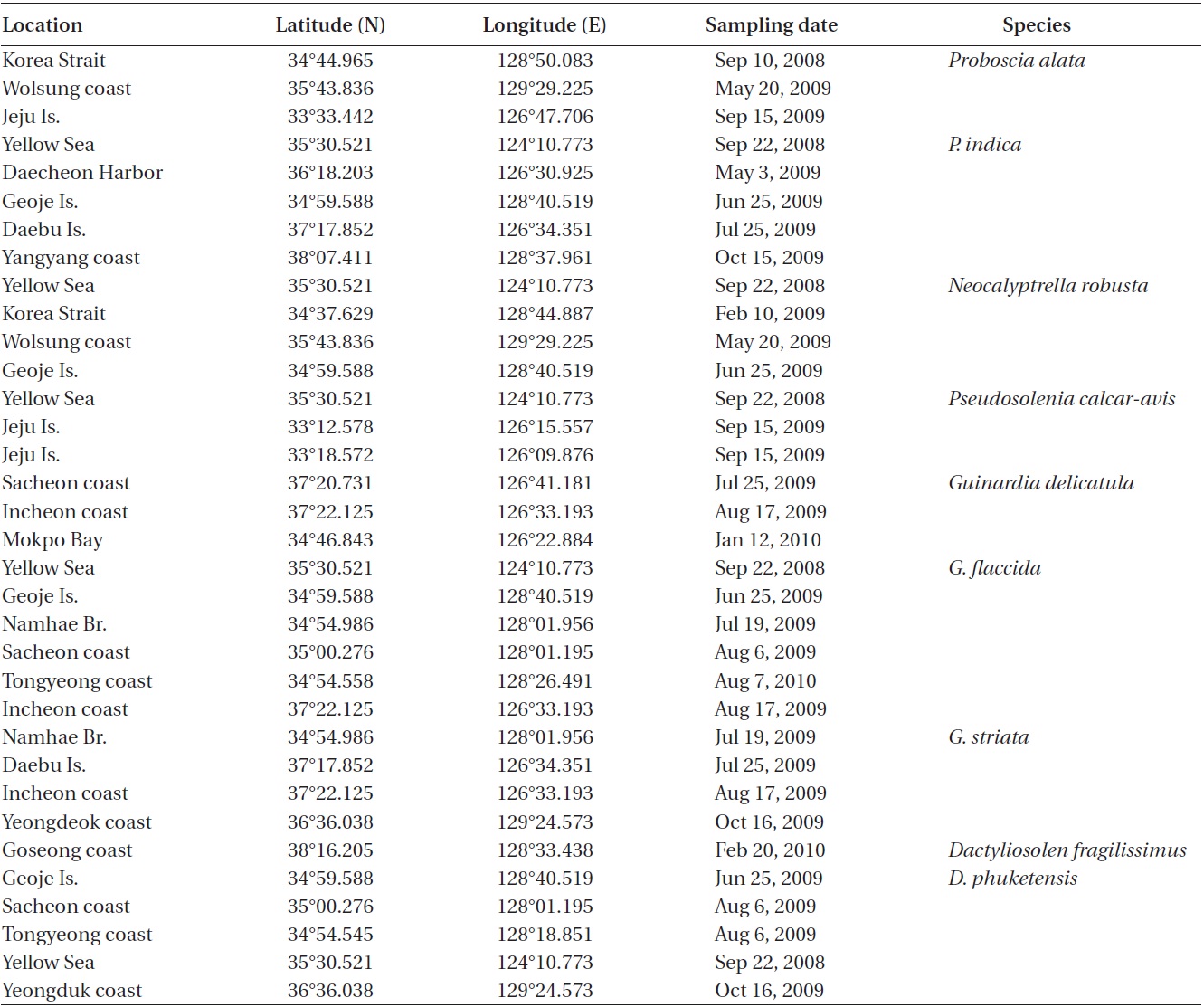
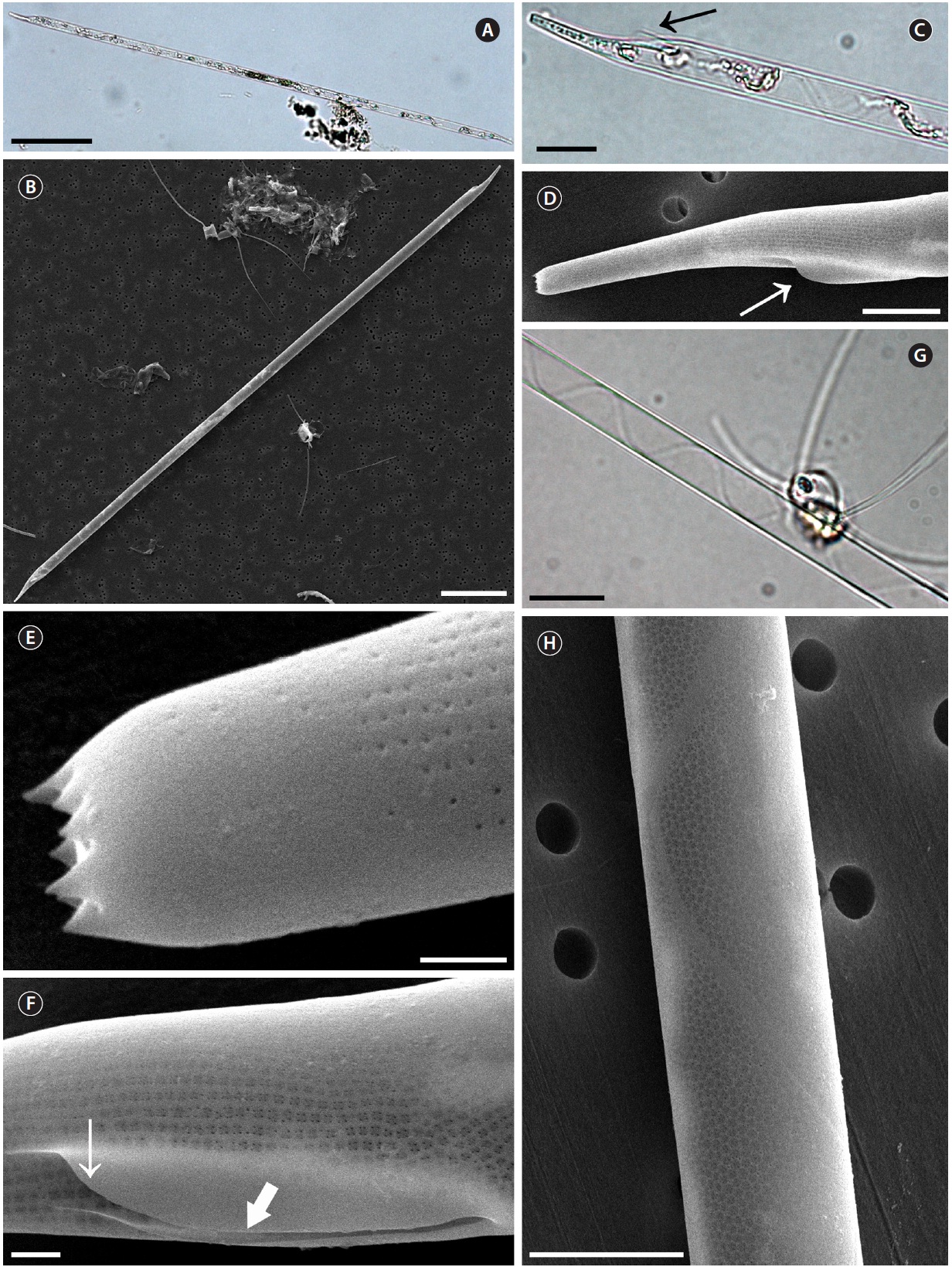
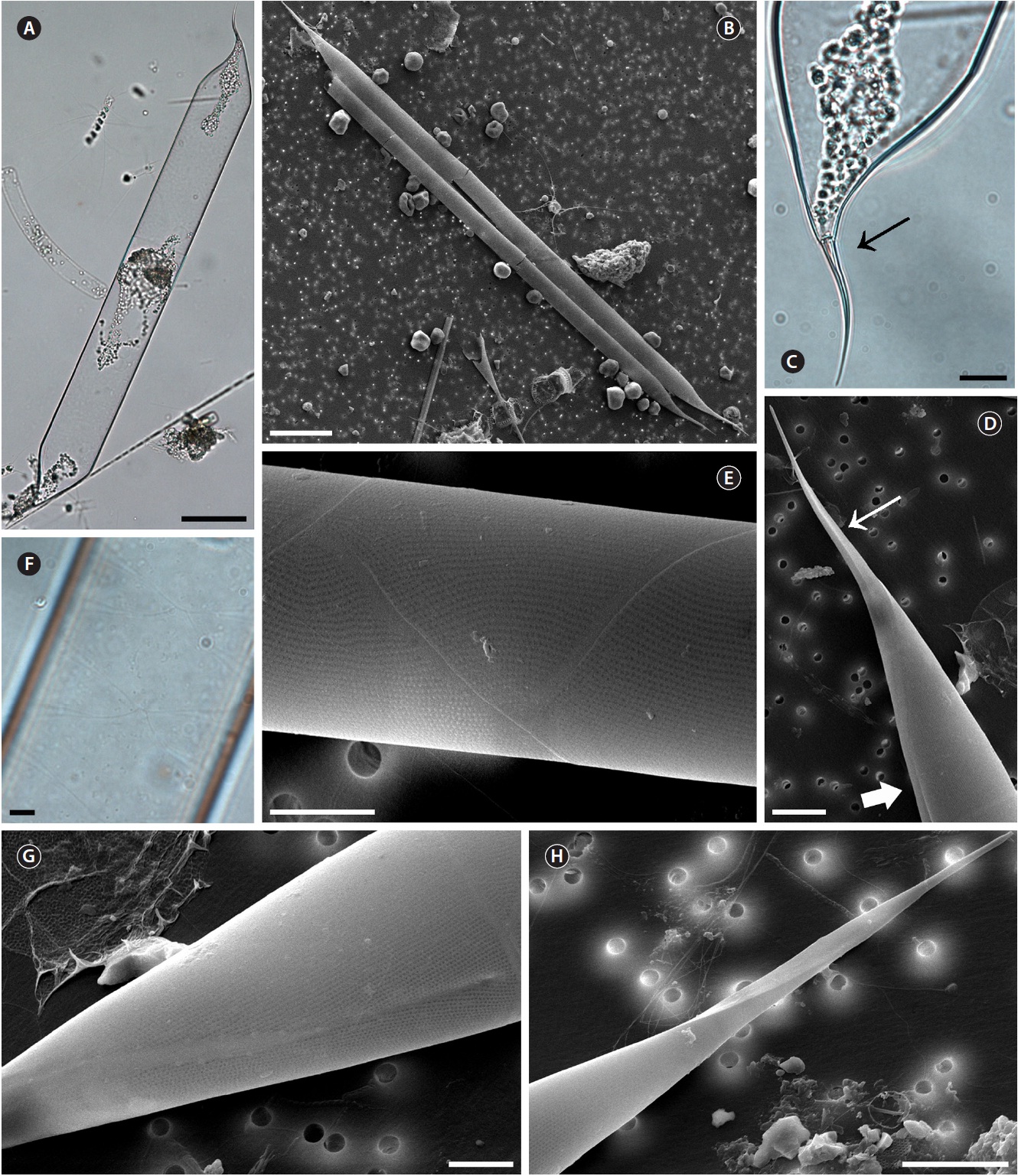
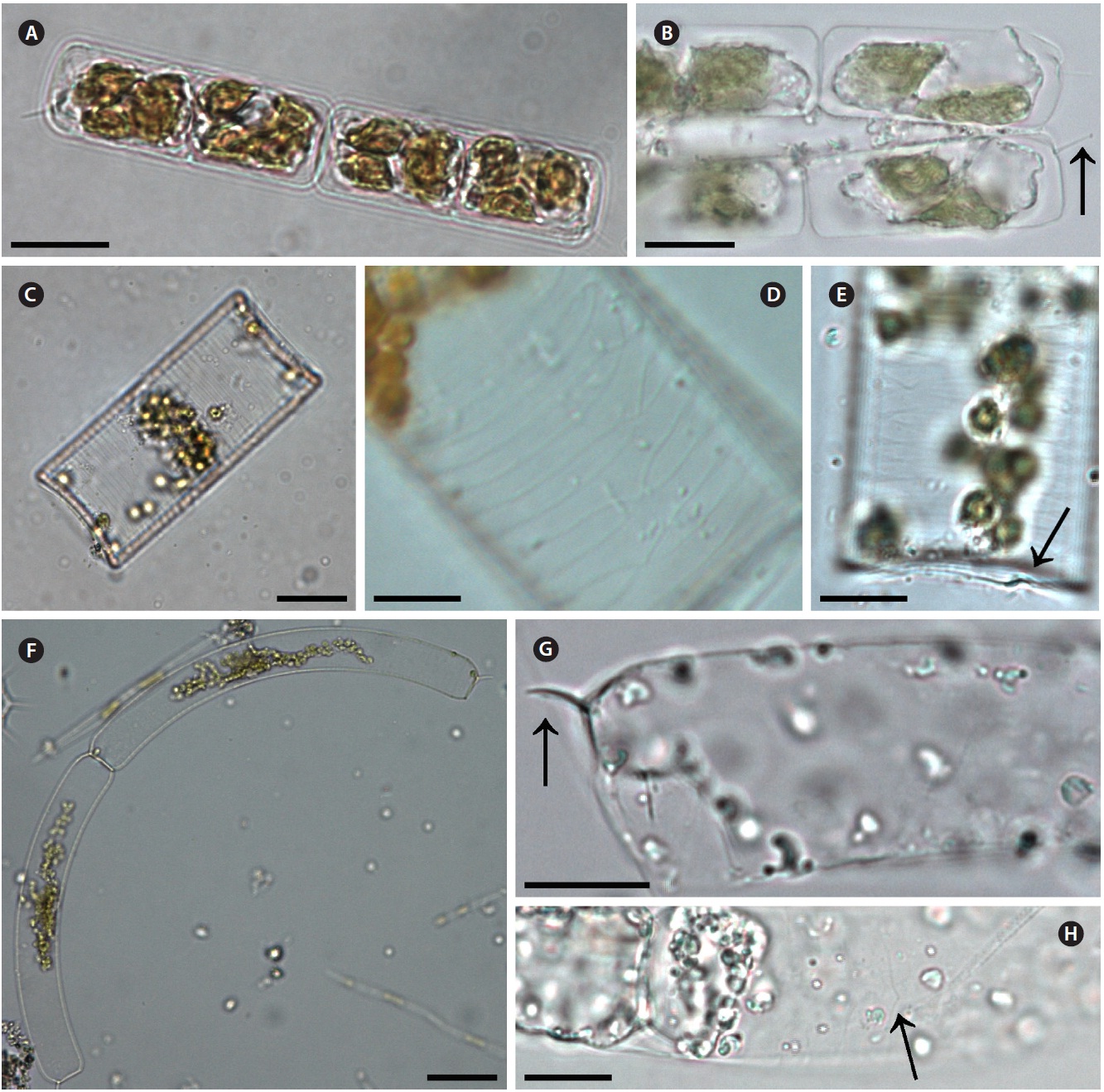
Cells are solitary or in pairs, narrow cylindrical, bilat-erally symmetrical, 3.3-13.3 μm in diameter, 270.0-485.7 μm long. Valve is sub-conoidal, the ventral part longer than the dorsal part and proboscis structure is slightly curved, tapering towards the apical part of the valve, cir-cular in cross section, 15.0-30.0 μm long. Apical surface of the proboscis is composed of variously sized spinules. Number of spinules is 7-16, 0.1-0.4 μm long. Contiguous area is convex towards the valve surface, distally limited by asymmetric claspers. The valve areolae are rounded, 52-90 in 10 μm, arranged in longitudinal striae, converg-
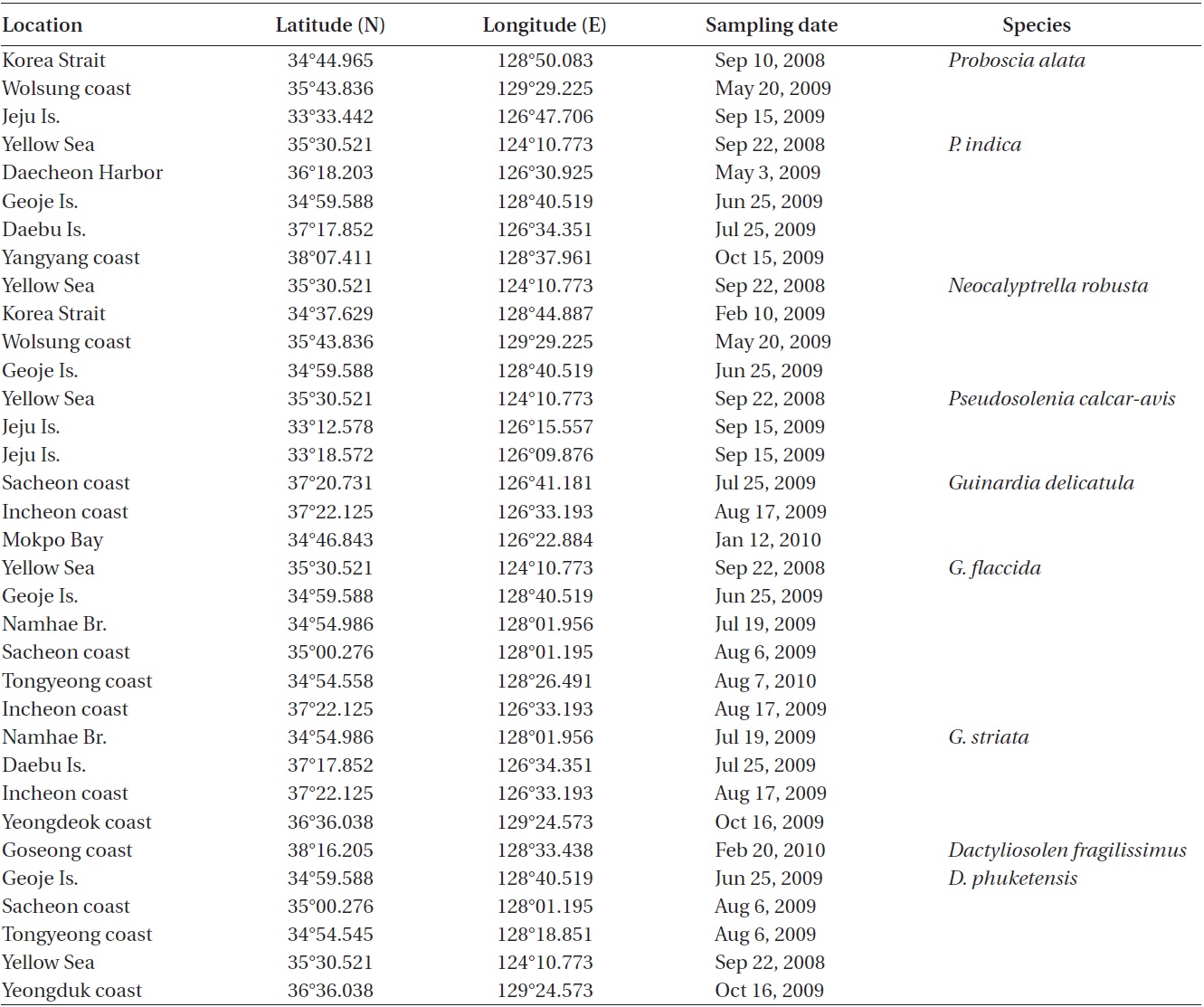
Sampling sites for the genera Proboscia, Neocalyptrella, Pseudosolenia, Guinardia, and Dactyliosolen of the family Rhizosoleniaceae
ing towards the apex. Girdle segment areolae are loculate, arranged in columns, with the external velum perforated by central pores, and internal circular foramina, 25-62 in 10 μm. Interlocular pores are commonly surrounded by six loculi. Segment horizontal axis and perpendicular axis are 3.3-13.3 and 10.0-26.7 μm long, respectively.
Distribution.
Remarks. Sundstrom (1986) did not share the biogeo-graphical limits of
[Table 2.] Morphological characteristics of the Proboscia species examined in this study
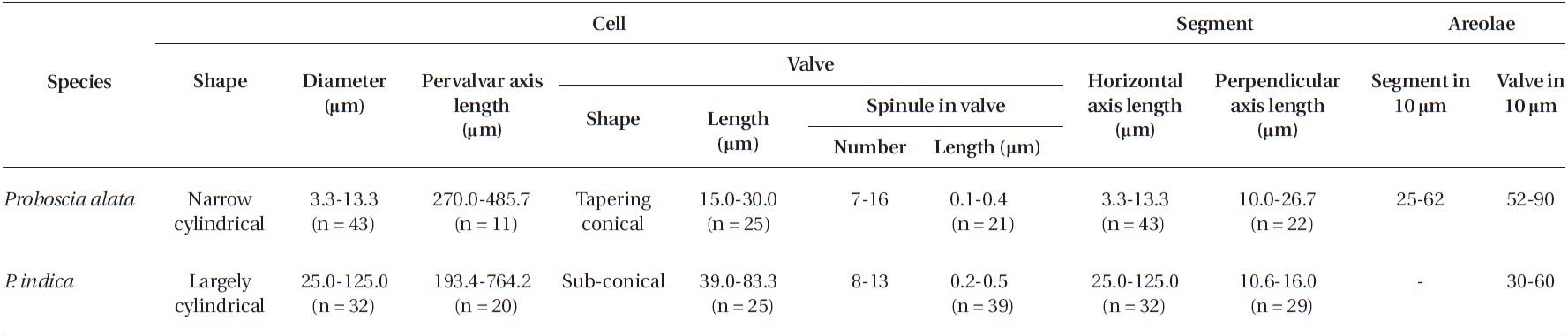
Morphological characteristics of the Proboscia species examined in this study
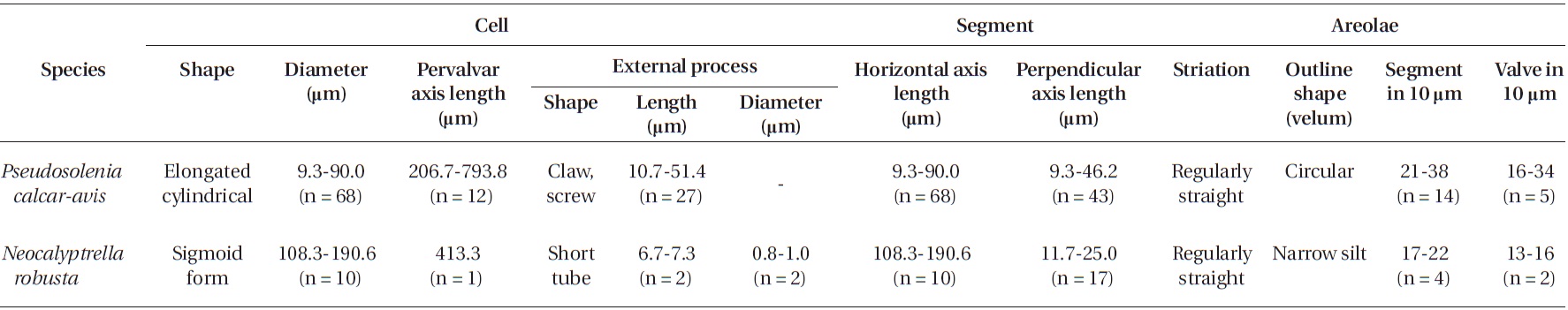
Morphological characteristics of the Pseudosolenia and Neocalyptrella species examined in this study
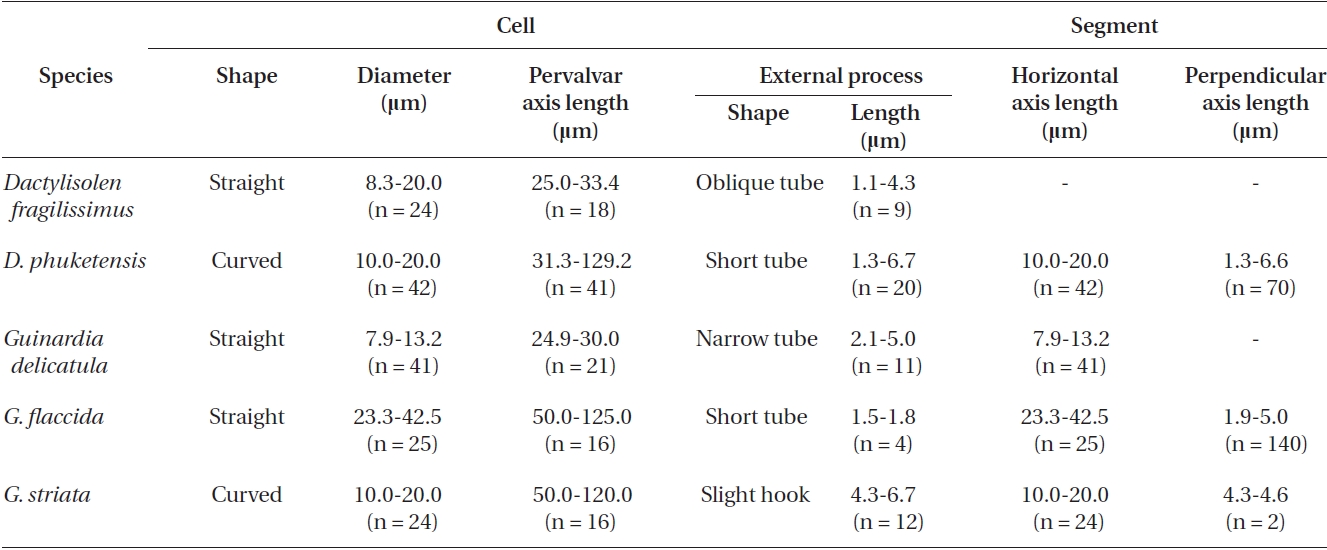
Morphological characteristics of the Dactylisolen and Guinardia species examined in this study
limits of the taxa including
>
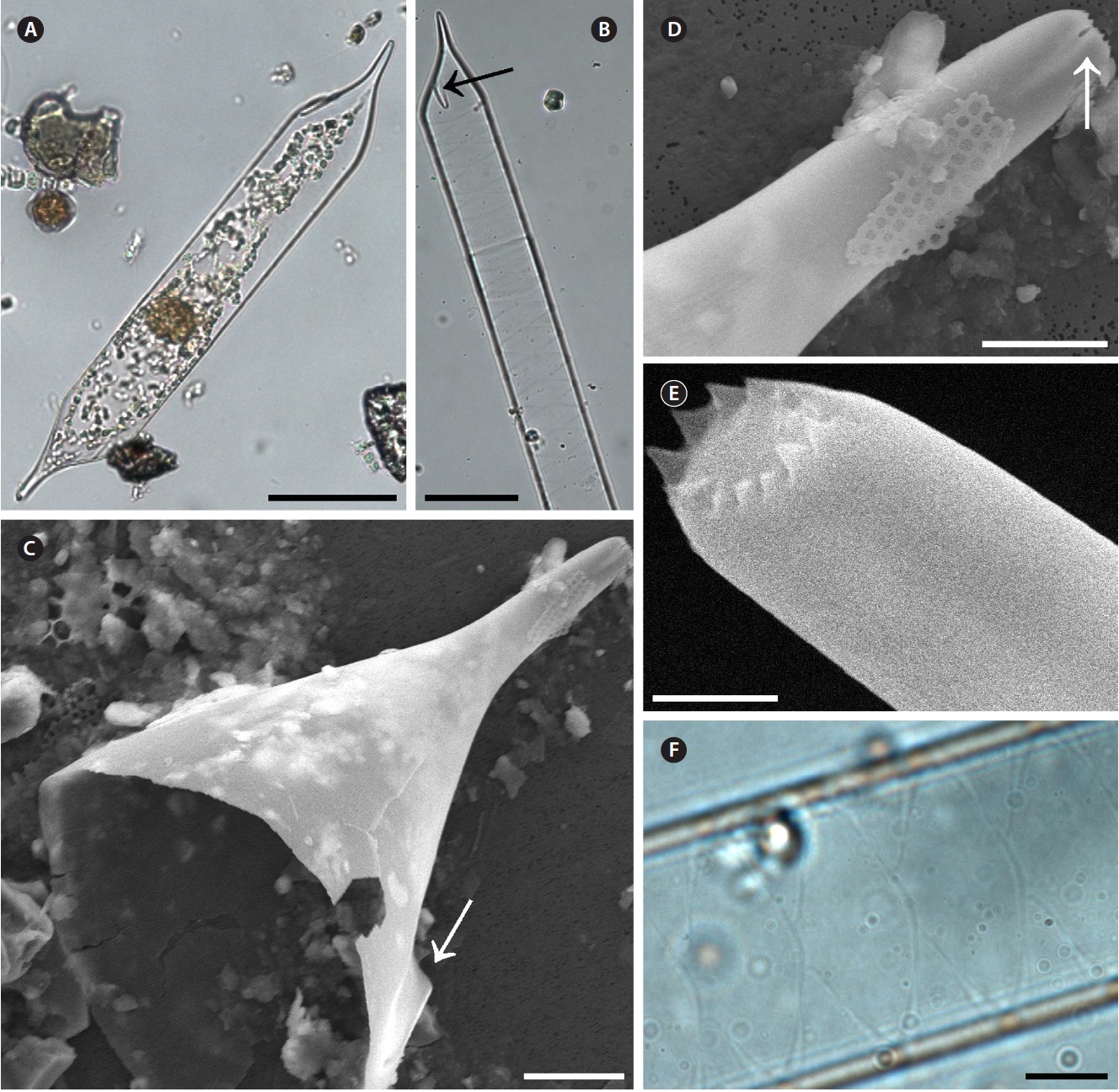
Proboscia indica (Peragallo) Hernandez-Becerril emend. Jordan & Ligowski 1995 (
Hustedt 1930, p. 602, Fig. 346; Cupp 1943, p. 93, Fig. 52C; Hendey 1964, p. 147, Pl. 2, Fig. 4; Hernandez-Becerril 1995, p. 254, Fig. 5 & 6; Moreno et al. 1996, p. 15, Pl. 29, Fig. 6 & 7; Jordan and Ligowski 2004, p. 98, Pl. 4, Fig. 5-7; Gomez and Souissi 2007, p. 287, Fig. 4g-h; Sunesen and Sar 2007, p. 639, Fig. 89-97 & 99.
Synonyms.
Cells are solitary or in pairs, cylindrical, bilaterally symmetrical, 25.0-125.0 μm in diameter, 193.4-764.2 μm long. Valve is sub-conoidal, the ventral part longer than the dorsal part. Proboscis structure is strongly curved, ta-pered towards the apical part in the valve, circular in cross section, 39.0-83.3 μm long. Apical surface of the proboscis is composed of varied sized spinules and the slit is pore shape situated below the apex. Spinule number is 8-13 and 0.2-0.5 μm long. Contiguous area is convex towards the valve surface, distally limited by asymmetric claspers. The valve areolae are rounded, 30-60 in 10 μm, arranged in longitudinal striae, converging towards the apex. Gir-dle segment areolae are loculate, arranged in columns, and the external velum is perforated by central pores and internal circular foramina. Interlocular pores are com-monly surrounded by four loculi. The horizontal axis of the segments is 25.0-125.0 μm and the perpendicular axis is 10.6-16.0 μm.
Distribution. Hendey (1964) reported that
Remarks.
>
Neocalyptrella robusta (Norman) Hernandez-Becerril & Meave 1996 (
Pritchard 1861, p. 866, Pl. 8, Fig. 42; Peragallo 1892, p. 109, Pl. 14, Fig. 1; Hustedt 1920, Pl. 320, Fig. 1-3; Hustedt 1930, p. 578, Fig. 330; Cupp 1943, p. 83, Fig. 46; Okuno 1957, p. 105, Pl. 2, Fig. 1; Okuno 1968, Fig. 1(6), 10A, 17 & 18; Navarro 1981, p. 430, Fig. 43 & 45; Sundstrom 1986, p. 104, Fig. 289 & 290 as
Synonyms.
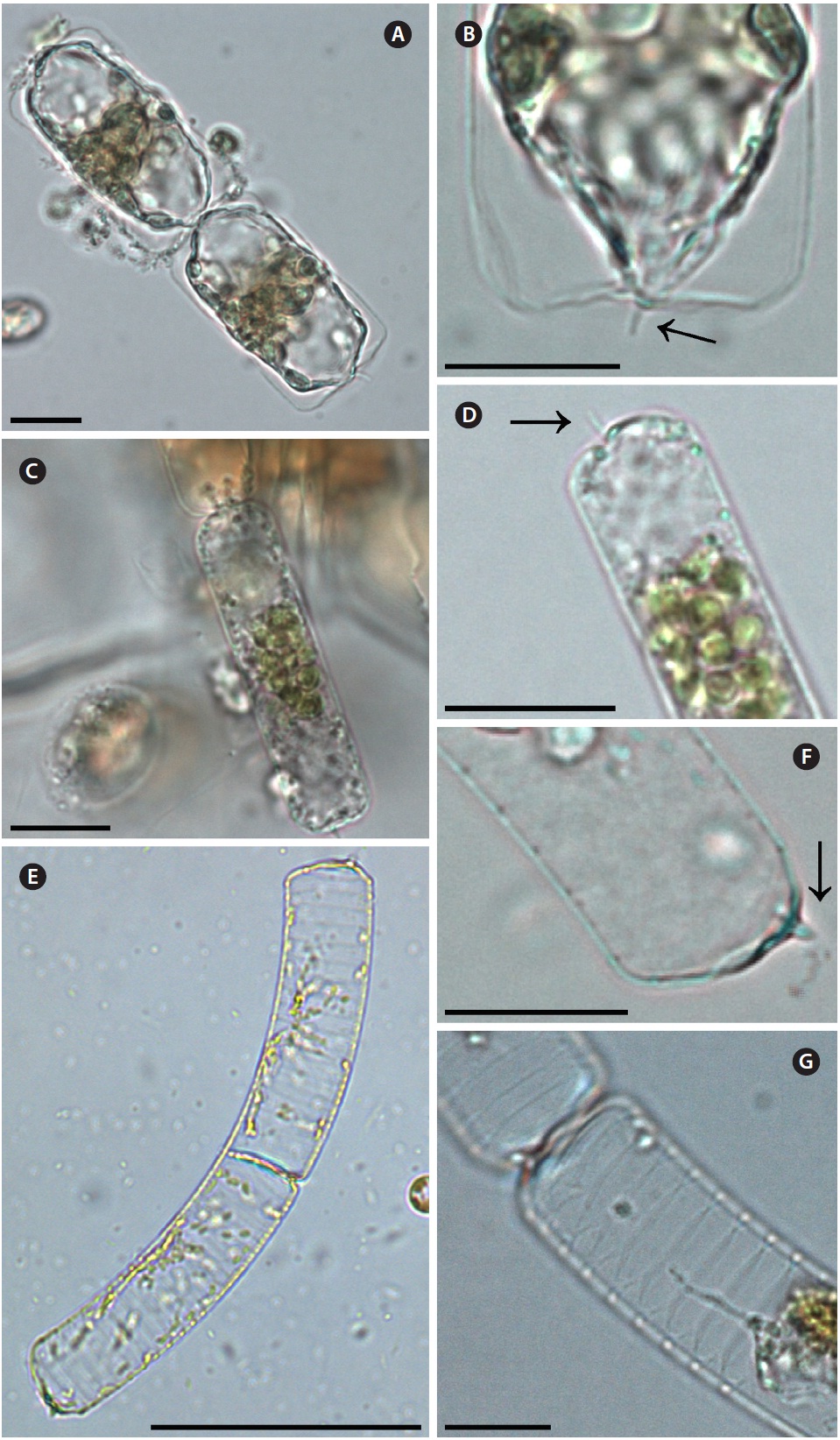
Cells are solitary, large, bilaterally symmetrical, 108.3-190.6 μm in diameter, 413.3 μm long, elliptical in cross sec-tion, crescent shaped in lateral view and of sigmoid form in ventro-dorsal view. Valve is conoidal with a rounded or truncated apex and with longitudinal undulations. Pro-cess is a cylindrical external tube, straightened towards the distal part, merging with the calyptra structure and circular pore in the distal part of the tip, 6.7-7.3 μm long, 0.8-1.0 μm in diameter. Valve areolae, 13-16 in 10 μm, are arranged in regularly straight striations, with a secondary quincuncial pattern. Otaria, claspers, and contiguous ar-eas are absent. Girdle segments are oriented in a straight line and arranged in two dorsiventral columns. Segment areolae, 17-22 in 10 μm, are arranged in regular, straight striations, with a secondary quincuncial pattern, loculate areolae, with the velum perforated by slit-like pores and internal foramina, circular to subcircular. Horizontal axis and perpendicular axis of segments are 108.3-190.6 and 11.7-25.0 μm in length, respectively.
Distribution.
>
Pseudosolenia calcar-avis (Schultze) Sundstrom 1986 (
Schultze 1858, p. 339, Pl. 13, Fig. 5-10; Peragallo 1892, p. 113, Pl. 17, Fig. 9; Hustedt 1930, p. 592, Fig. 339 as
Synonym.
Cells are usually solitary, elongated, of cylinder shape, bilaterally symmetrical, circular in cross section, 9.3-90.0 μm in diameter, 206.7-793.8 μm long. Valve is sub-conical, asymmetrical, with the ventral part slightly longer than the dorsal part. Contiguous area is a narrow groove, sig-moid, extended from the basal part of the process to the margin in the ventral part of the valve. Process is claw or screw shaped, slightly or strongly curved, and tapered towards the distal part, 10.7-51.4 μm long. Otaria and claspers are absent. Valve areolae are poroid, circular, 16-34 in 10 μm. Striations are regular and straight, with a secondary quincuncial pattern. Girdle segments are scale-shaped to rhomboidal, arranged in two or multiples of two columns, with a sub marginal seam-like structure close to the advalvar margin with entire hyaline edges. Horizontal axis and perpendicular axis of segments are 9.3-90.0 and 9.3-46.2 μm long, respectively. Segmented areolae are 21-38 in 10 μm in a secondary quincuncial pattern.
Distribution.
>
Guinardia delicatula (Cleve) Hasle 1995 (
Cleve 1900, p. 28, Fig. 11; Hustedt 1930, p. 577, Fig. 328; Cupp 1943, p. 83, Fig. 44; Hendey 1964, p. 147, Pl. 4, Fig. 2; Drebes 1974, p. 49, Fig. 35a; Sundstrom 1986, p. 103, Fig. 272 & 273.
Basionym.
Cells form fairly straight chains and are bilaterally sym-metrical. Cells are 7.9-13.2 μm in diameter, 24.9-30.0 μm in length. Valve margins are round. External process is thin and short, and narrow, tube-shaped, and oblique to the pervalvar axis. External processes are 2.1-5.0 μm long. External process fits into a depression on the adjacent valve. Girdle segments are composed of open bands, with poroid areolae, and are not noticeable. Segment horizon-tal axes are 7.9-13.2 μm long.
Distribution. Hasle and Syvertsen (1996) reported that
>
Guinardia flaccida (Castracane) H. Peragallo 1892 (
Castracane 1886, p. 74, Pl. 29, Fig. 4; Peragallo 1892, p. 107, Pl. 1, Fig. 3-5; Bergon 1903, p. 78, Pl. 2, Fig. 1-3; Hustedt 1930, p. 562, Fig. 322; Cupp 1943, p. 78, Fig. 40; Hendey 1964, p. 147, Pl. 5, Fig. 5; Drebes 1974, p. 58, Fig. 43a; Hasle 1975, p. 116, Fig. 64, 65 & 81-89; Navarro 1981,
p. 430, Fig. 31 & 32; Takano 1990, pp. 260-261.
Basionym.
Cells are solitary or form fairly straight chains, and are bilaterally symmetrical. Cells are 14.0-42.5 μm in diame-ter, 50.0-125.0 μm in length. Valves are flat or slightly con-cave. External processes are short and tube-shaped. Short tube-shaped external processes are located on the exter-nal valve surface. External processes are 1.5-1.8 μm long. Girdle segments composed of open bands with poroid areolae. The segment horizontal axis and perpendicular axis are 14.0-42.5 and 1.3-5.0 μm in length, respectively.
Distribution.
>
Guinardia striata (Stolterforth) Hasle 1995 (
Stolterforth 1879, p. 836, Fig. a & b; Peragallo 1888, p. 82, Pl. 6, Fig. 44; Peragallo 1892, p. 108, Pl. 13, Fig. 17 & 18; Bergon 1903, p. 57, Pl. 1, Fig. 1-8; Hustedt 1920, Pl. 320, Fig. 4 & 5; Hustedt 1930, p. 578, Fig. 329; Cupp 1943, p. 83, Fig. 45; Hendey 1964, p. 148, Pl. 4, Fig. 5; Drebes 1974, p. 49, Fig. 35b; Hasle 1975, p. 113, Fig. 66-73; Sundstrom 1980, p. 580, Fig. 2-4; Navarro 1981, p. 430, Fig. 48 as
Basionym.
Cells form curved chains, rarely spiraling chains, and are bilaterally symmetrical. Cells are 10.0-20.0 μm in diameter, and 50.0-120.0 μm in length. Valve flat and rounded at margin. External processes are thin, and hook-shaped to the pervalvar axis, and the external pro-cesses are 4.3-6.7 μm long. External process fits into a de-pression on the adjacent valve. Girdle bands composed of open bands with poroid areolae. Segment horizontal axis and perpendicular axis are 10.0-20.0 and 4.3-4.6 μm long, respectively.
Distribution.
>
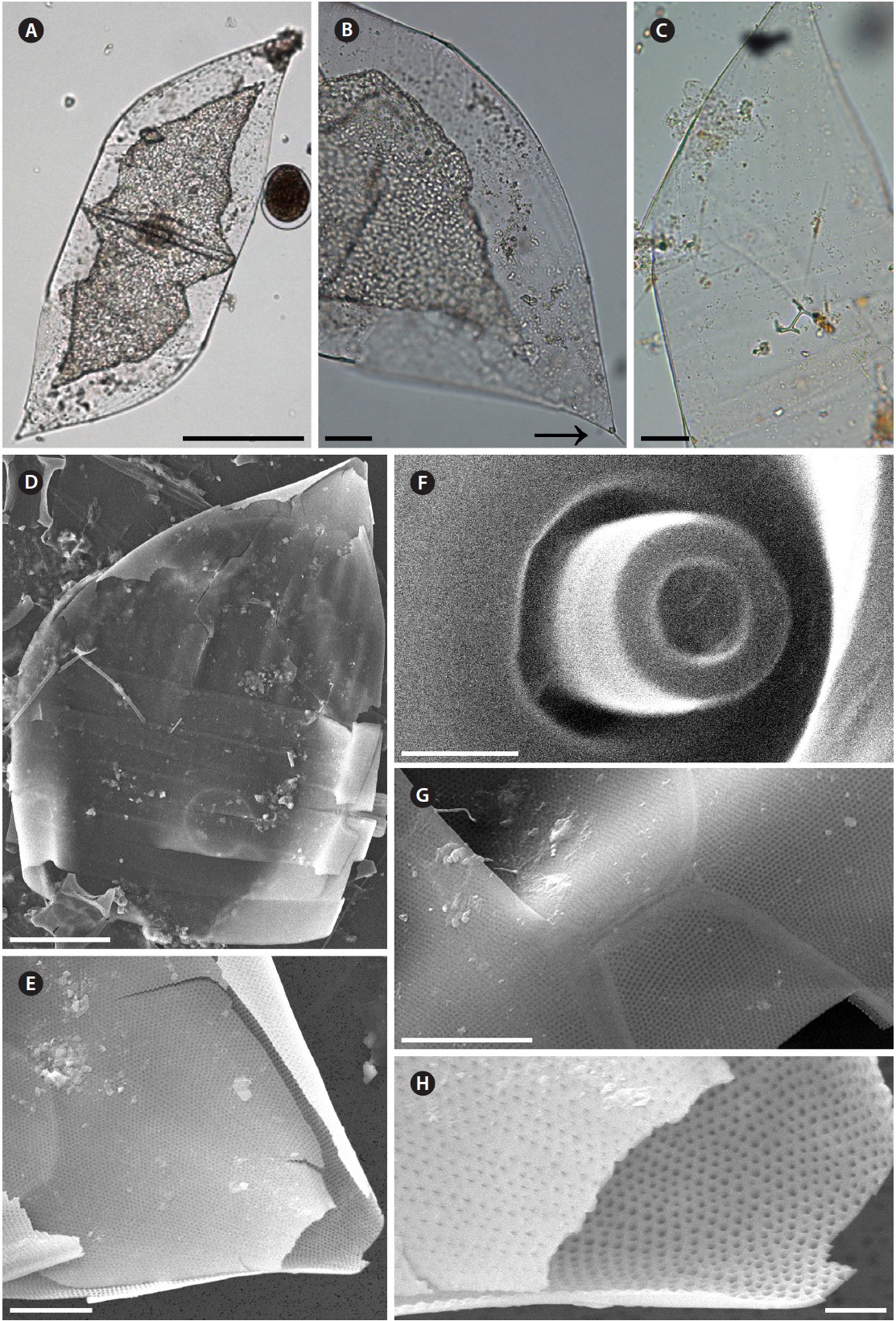
Dactyliosolen fragilissimus (Bergon) Hasle 1995 (
Bergon 1903, p. 49, Pl. 1, Fig. 9 & 10; Hustedt 1930, p. 571, Fig. 324; Cupp 1943, p. 80, Fig. 41; Drebes 1974, p. 48, Fig. 34b & c; Hasle 1975, p. 114, Fig. 61, 62 & 74-78; Navarro 1981, p. 430, Fig. 38 as
Basionym.
Cells are cylindrical with rounded marginal parts, forming straight chains. Cells are connected in loose fit-ting chains at the center of the valve surface. Cells are 8.3-20.0 μm in diameter, and 25.0-33.4 μm in length. Valves are flat or convex at the central part. External process is a thin, oblique tube in the central part of the valve. External processes are 1.1-4.3 μm long and fit into a depression on adjacent cells. Girdle bands composed of half bands with poroid areolae.
Distribution.
Remarks. Cell length of
>
Dactyliosolen phuketensis (Sundstrom) Hasle 1995 (
Sundstrom 1980, p. 579, Fig. 1-3; Sundstrom 1986, p. 103, Fig. 270 & 271; Von Stosch 1986, p. 323, Fig. 15-17; Hernandez-Becerril 1995, p. 262, Fig. 50-52.
Basionym.
Cells are cylindrical with a rounded marginal part, forming curved chains. Cells are connected in fairly fitting chains and are bilaterally symmetrical. Cells are 10.0-20.0 μm in diameter, and 31.3-129.2 μm long. Valves are flat or slightly convex. External process is usually an obtuse, short tube in the valve marginal part. External processes are 1.3-6.7 μm long and fit into a depression in adjacent cells. Girdle bands are composed of half bands with po-roid areolae. Segment horizontal axis and perpendicular axis are 10.0-20.0 and 1.3-6.6 μm in length, respectively.
Distribution. Sundstrom (1986) reported that
Remarks.
The family Rhizosoleniaceae includes
As shown in Table 2 of Yun and Lee (2010), Table 2 in Yun et al. (2011), and Table. 2-4 in the present study, we divided the 6 genera within the family Rhizosoleniaceae into two groups by morphological diagnostic characteris-tics including the shape of the external process and girdle segments in the column (Yun and Lee 2010, Yun et al. 2011). The first group had a conoidal valve and loculate areolae and was comprised of
Cell diameters of
Cell diameters of
Cell diameters of
External processes varied from short tube-shaped in
Areolae occurred in various forms on the external view; circular to sub circular pore-shaped in
The number of areolae in the valves varied from 52-90 in 10 μm in
Abundant distributions of