The brown seaweed
Fermentation is used in plant foods to increase the nutritional quality and remove undesirable compounds (Frias et al. 2005). Several microbial fermentation prod-ucts are also incorporated into food as additives and supplements such as antioxidants, flavors, colorants, pre-servatives, and sweeteners (Couto and Sanroman 2006). Microorganism-fermented foods play an important role, particularly in Asian countries, where the production process for many foods includes fungal fermentation (Geisen and Farber 2002). Microorganisms play a central role in the production of a wide range of primary and sec-ondary metabolites.
In this study, we used enzyme-assisted extraction (EAE) followed by fermentation to improve extraction efficiency of the active polysaccharides in
Murine macrophage cells such as RAW 264.7 cells play a central role in the inflammatory response and serve as an essential interface between innate and adaptive im-munity (Iontcheva et al. 2004). During the inflammatory process, large amounts of the proinflammatory media-tor nitric oxide (NO) are generated by inducible isoforms of NO synthase (iNOS) and cyclooxygenase-2 (COX-2) (Vane et al. 1994). Additionally, abnormal NO produc-tion can be deleterious and has been implicated in the pathogenesis of various inflammatory diseases (Yun et al. 1996). For these reasons, inhibiting NO production is important to reduce the pathogenesis of various inflam-matory diseases.
Therefore, the present study documented whether co-applying EAE and fermentation would improve the extraction yield of polysaccharide contents from
>
Preparation of fermented E. cava (FE) samples
>
Chemical composition analysis
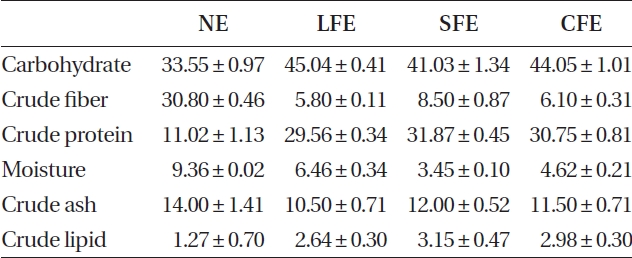
The chemical composition of all samples was analyzed by measuring the contents of carbohydrate, protein, ash, and / or lipid from the weight difference after drying sam-ples, according to the methods described by Association of Official Analytical Chemists.
>
Preparation of aqueous extracts from NE, LFE, SFE, and CFE
Aqueous extracts were prepared from NE, LFE, SFE, and CFE. One gram of freeze-dried sample was homog-enized in distilled water (100 mL). After 24 h, the samples were obtained and kept at -20℃ for further experiments.
>
Preparation of the LFE enzymatic extracts
LFE was used for EAE with several enzymes according to a previously reported method (Heo et al. 2005). Fifty grams of LFE was homogenized in distilled water (2 L) with 500 μL of 10 kinds of enzymes separately. Each re-actant was adjusted to the optimum pH and temperature range of the respective enzyme, and enzymatic reactions were performed for 24 h. Following digestion, the digest was boiled for 10 min at 100℃ to inactivate the enzymes. After centrifugation (3,000 rpm for 20 min at 4℃), the su-pernatant was adjusted to pH 7.0. The samples were kept at -20℃ for further experiments.
>
Preparation of molecular weight fractions from the Viscozyme extract of LFE (VLFE)
Extracts were applied to a lab-scale tangential flow fil-tration system (Millipore, Billerica, MA, USA) using an ul-tra-filtration membrane (30 kDa) to prepare different mo-lecular weight fractions Then, all fractions (whole extract, <30 kDa fraction, and >30 kDa fraction) were separately evaluated for their NO inhibitory effects.
>
Isolation of a polysaccharide from the >30 kDa fraction of VLFE (VLFEP)
VLFEP was isolated from a >30 kDa VLFEP by ethanol precipitation according to a slightly revised method from a previous study (Athukorala et al. 2009). The >30 kDa VLFE fraction (1 L) was mixed with 2 L of 99.5% ethanol for 24 h at 4℃. After centrifugation at 10,000 rpm for 20 min at 4℃, crude polysaccharides were collected from the precipitant. Then, the crude VLFEP was freeze-dried and used for subsequent experiments.
>
Analysis of mono-sugar and sulfate group contents
VLFE, and the >30 kDa and <30 kDa fractions as well as VLFEP were hydrolyzed in a sealed glass tube with 4 M trifluoroacetic acid for 4 h at 100℃ to analyze neutral sugar content. The samples were digested using 6 N of HCl for 4 h to analyze mono-sugars. Then, the samples were separately applied to a CarboPac PA1 column (4.5 × 250 mm; Dionex, Sunnyvale, CA, USA) with a CarboPac PA1 cartridge (4.5 × 50 mm), respectively. The column was eluted using 16 mM NaOH at a 1.0 mL min-1 flow rate. Each sugar was detected using an ED50 Dionex electro-chemical detector, and data were analyzed using Peak Net on-line software.
RAW 264.7 cells (murine macrophage cell line) were plated at a density of 1 × 105 cells well-1 in 96-well plates for 16 h. The cells were pre-treated with aqueous and 10 enzymatic extracts of LFE, <30 kDa, and >30 kDa frac-tions of VLFE and VLFEP for 2 h and then stimulated with LPS (1 μg mL-1) for 24 h. After the incubation, the culture media (100 μL well-1) was mixed with 100 μL Griess re-agent, an indicator of NO production (1% sulfanilamide in 2.5% phosphoric acid and 0.1% naphthylenediamine dihydrochloride in distilled water), for 10 min, and the absorbance of the mixture was measured at 540 nm using a microplate reader (Amersham Pharmacia Biotech, Pis-cataway, NJ, USA). The nitrite levels were obtained from a sodium nitrite standard curve. The experimental results represented one of three experiments and were expressed as the mean of triplicate determinations.
Data were analyzed using SPSS for Windows version 10 (SPSS Inc., Chicago, IL, USA). Values are expressed as means ± standard errors. A p-value < 0.05 was considered significant.
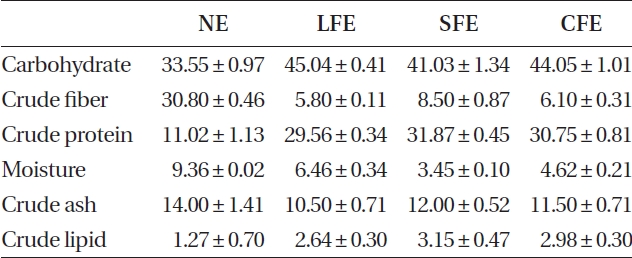
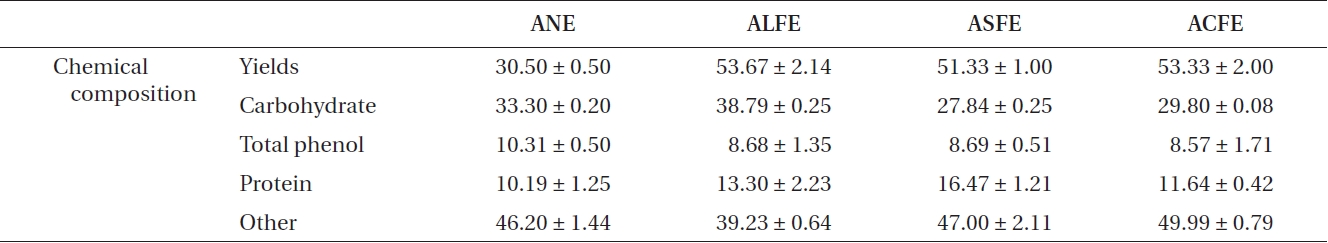
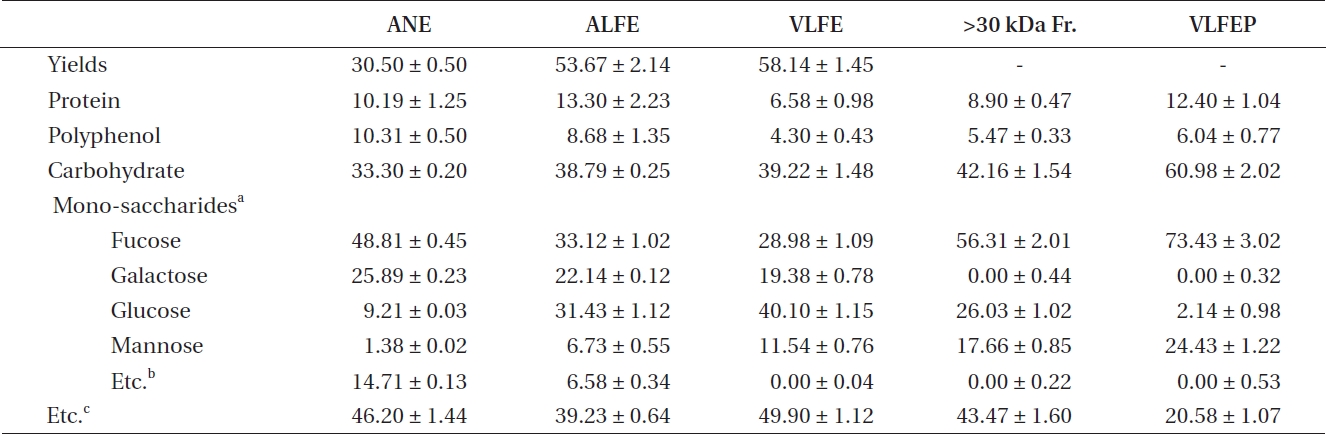
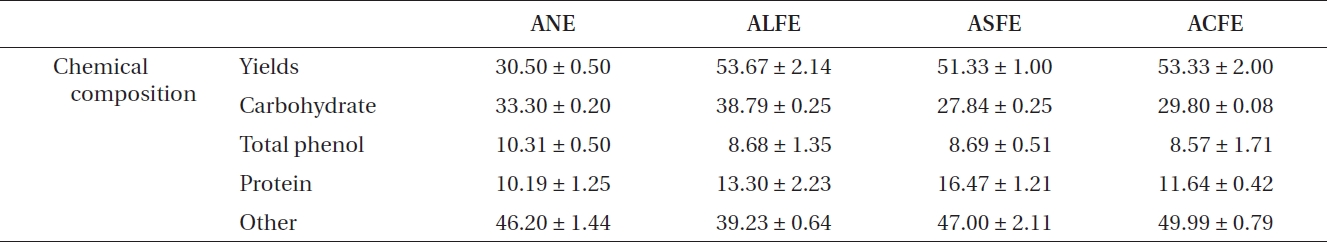
The three FEs showed plentiful carbohydrate and pro-tein content after fermentation (Table 1). In contrast, we identified three FE samples in which the carbohydrate content increased slightly, but their protein content in-creased markedly, compared to that of NE. Increased ex-traction yields were observed for aqueous extract of LFE (ALFE), aqueous extract of SFE (ASFE), and aqueous ex-tract of CFE (ACFE) (Table 2). Additionally, carbohydrate content increased markedly following fermentation. Among them, ALFE showed the highest carbohydrate content and extraction yield. Further study showed that ALFE, ASFE, and ACFE led to higher NO production in-
[Table 1.] Chemical composition of NE, LFE, SFE, and CFE
Chemical composition of NE, LFE, SFE, and CFE
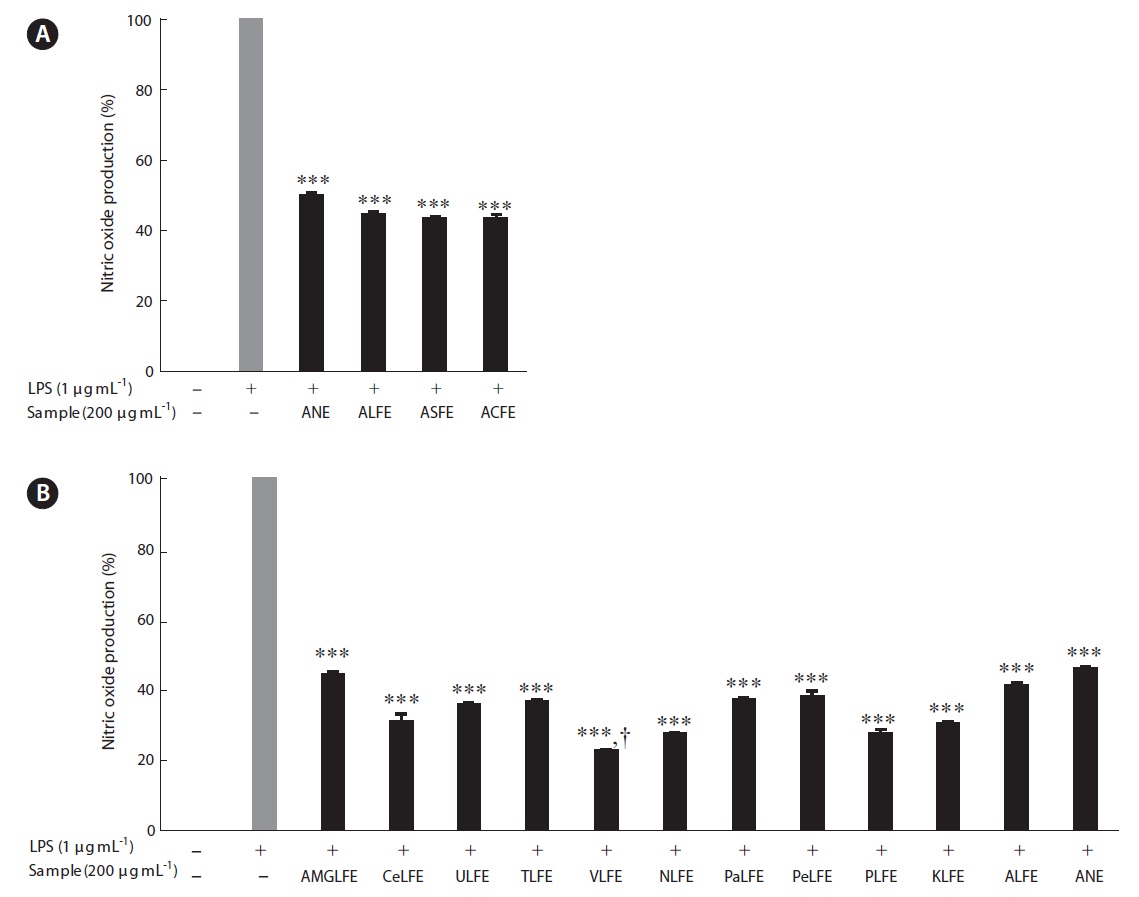
hibitory effects in LPS stimulated RAW 264.7 cells com-pared to those treated with aqueous extract of NE (ANE) (Fig. 1A). However, the NO inhibitory effects were similar together. Therefore, we selected LFE for the next experi-
[Table 2.] Chemical composition and extraction yield of ANE, ALFE, ASFE, and ACFE
Chemical composition and extraction yield of ANE, ALFE, ASFE, and ACFE
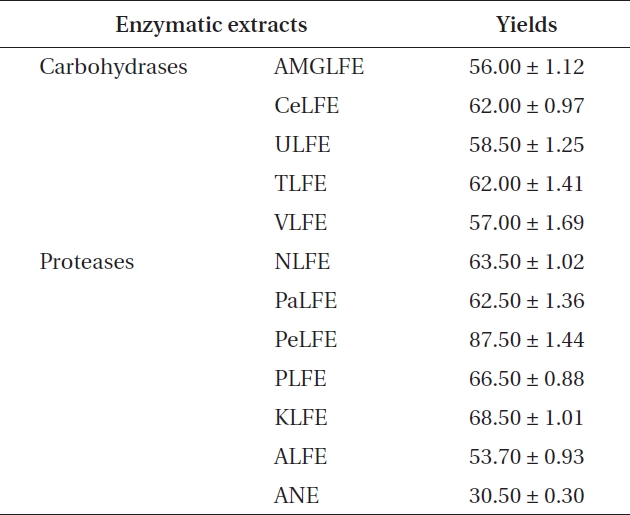
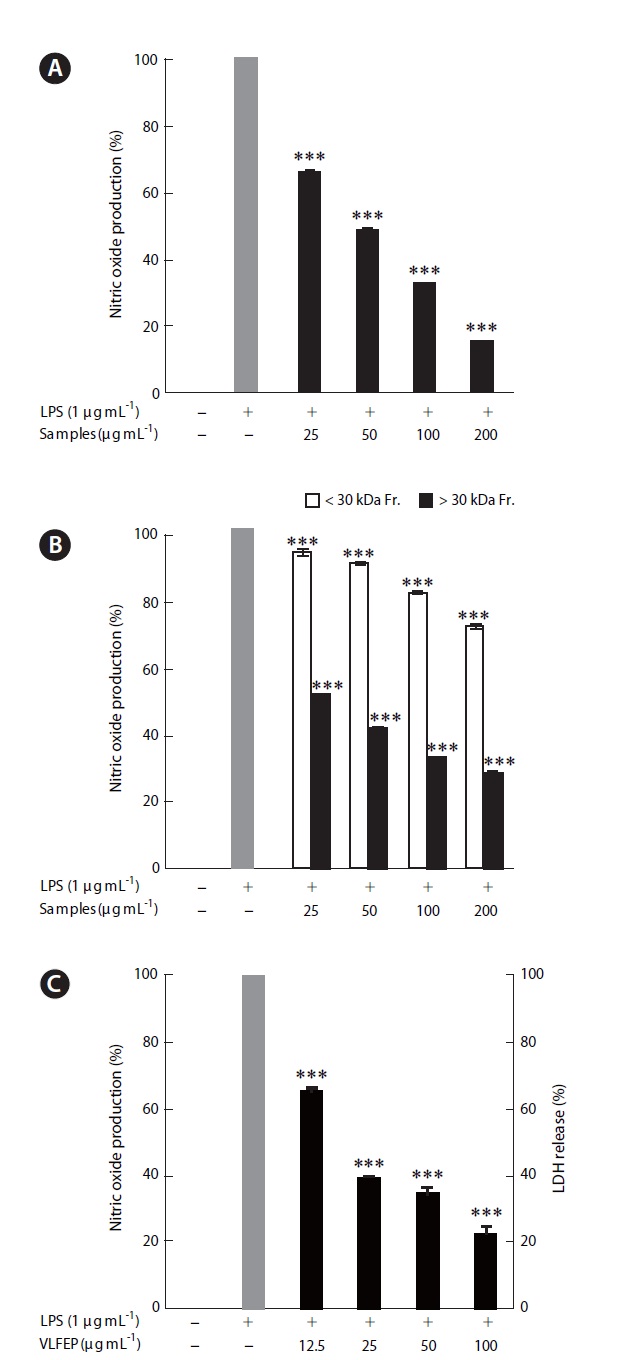
ments, because it showed the highest extraction yield and carbohydrate content. In the next experiment, 10 LFE en-zymatic extracts were prepared using five carbohydrates and five proteases. Markedly increased extraction yields were obtained from all enzymatic extract treatments (Ta-ble 3). In addition, LFE enzyme extracts strongly inhibited the NO production induced by LPS stimulation in RAW 264.7 cells (***p < 0.005 vs. LPS-treated RAW 264.7 cells) (Fig. 1B). Among them, celluclast extract of LFE, VLFE, neutrase extract of LFE, protamex extract of LFE, and ko-jizyme extract of LFE showed higher inhibitory effects on NO production induced by LPS stimulation in RAW 264.7 cells compared to those of ANE and ALFE (Fig. 1B). In particular, VLFE showed the highest inhibitory effect on NO production in LPS-treated RAW 264.7 cells (†p < 0.005 vs. the other LFE enzyme extracts and LPS-treated RAW 264.7 cells). Moreover, VLFE dose-dependently decreased NO production at all concentrations from 25-200 μg mL-1 in comparison with that in LPS-treated cells (***p < 0.005 vs. LPS-treated RAW 264.7 cells) (Fig. 2A).
As shown in Fig. 2B, both fractions (<30 kDa fraction and >30 kDa fraction) decreased the NO production in-duced by LPS stimulation and, particularly, the >30 kDa fraction led to a markedly high inhibitory effect on LPS-induced NO production, as compared to that of the <30 kDa fraction at all concentrations (***p < 0.005 vs. LPS-treated RAW 264.7 cells).
The crude polysaccharide isolated from VLFE (VLFEP) showed the highest carbohydrate content among the oth-
[Table 3.] Extraction yields of ANE and 10 enzymatic extracts pre-pared from LFE
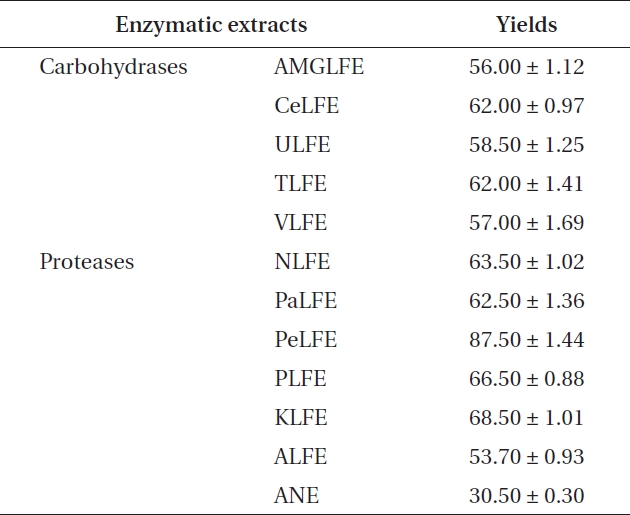
Extraction yields of ANE and 10 enzymatic extracts pre-pared from LFE
ers (Table 4). Additionally, fucose and mannose contents increased with the increase in carbohydrate content in VLFE. In particular, VLFEP showed the highest fucose
[Table 4.] Mono-saccharide contents of ANE, VLFE, its >30 kDa fraction and VLFEP
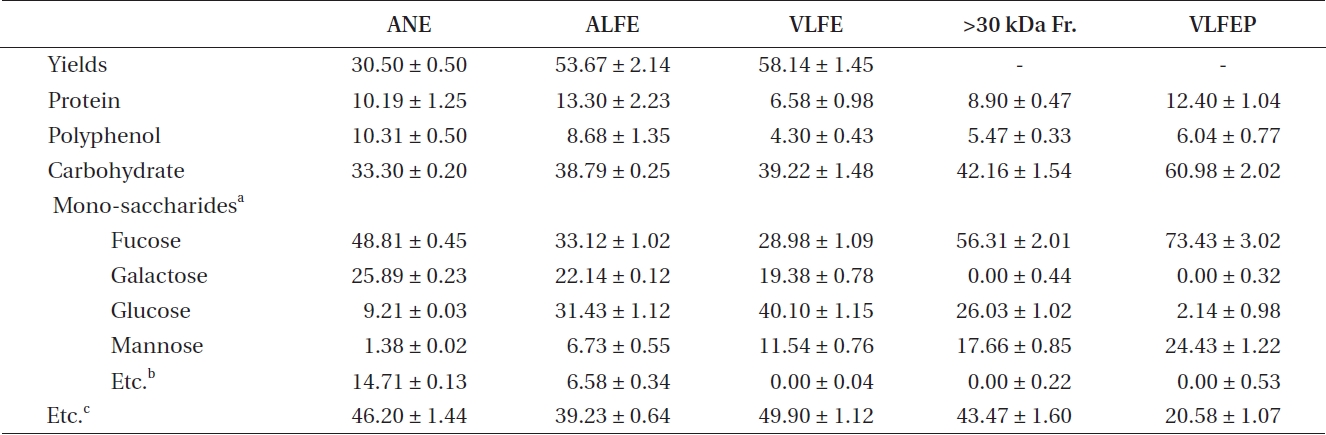
Mono-saccharide contents of ANE, VLFE, its >30 kDa fraction and VLFEP
content among VLFE and its >30 kDa fraction. Further-more, VLFEP was not cytotoxic at any concentration and dose-dependently induced a higher inhibitory effect on NO production caused by LPS stimulation in Raw 264.7 cells (***p < 0.005 vs. LPS-treated RAW 264.7 cells) (Fig. 2C). Interestingly, the NO inhibitory effect of the samples in LPS-stimulated Raw 264.7 cells corresponded to the in-crease in fucose content.
Many researchers have reported that fermented mate-rials have immune response and oxidative stress activi-ties
Our previous studies reported that
An ultra-filtration system using a 30 kDa molecular weight cut-off membrane is another technique for isolat-ing specific hydrophilic compounds such as saccharides. In this study, we also applied an ultra-filtration process and obtained two molecular weight fractions from VLFE. Polysaccharides from natural sources are a class of macro-molecules that can profoundly affect the immune system and, therefore, have the potential to be immunomodula-tors with wide clinical applications (Tzianabos 2000). Pre-vious studies have indicated that various polysaccharides show beneficial antiinflammatory effects such as NO in-hibition (Cui et al. 2010, Jung et al. 2010). Our results sug-gest that VLFEP might be an active compound exhibiting NO inhibition effects in RAW 264.7 cells.
Therefore, we first isolated a crude polysaccharide fraction from the >30 kD fraction. Then, we identified the chemical composition including protein, polyphenols, carbohydrates, and the mono-sugar content such as fu-cose, galactose, glucose, and mannose which are stan-dard components of polysaccharides.
Our results demonstrated that co-applying fermen-tation, EAE, and an ultra-filtration membrane system improved extraction of fucose and lead to a decrease in NO production in LPS stimulated Raw 264.7 cells. Many studies have reported that NO is generated by iNOS and COX-2 during inflammatory processes, and that abnor-mal NO production causes the pathogenesis of various inflammatory diseases (Vane et al. 1994, Yun et al. 1996). Hence, inhibiting NO production is important to reduce the pathogenesis of various inflammatory diseases. NO, COX-2, and iNOS are regulated by a variety of inflamma-tory cytokines including tumor necrosis factor-α, inter-leukin (IL)-1β, and IL-6 in LPS-activated RAW 264.7 cells (Chang et al. 2005). Moreover, many researchers have demonstrated that the transcription and secretion of pro-inflammatory mediators and cytokines are mediated by activation of the IκB / nuclear transcription factor kappa-B (NFκB) signal transduction pathway in LPS-activated RAW 264.7 cells (Surh et al. 2001, Lappas et al. 2002). This suggest that the NO inhibition effects of VLFEP might be mediated by reducing pro-inflammatory mediators and cytokines via inhibition of the NFκB signal transduction pathway activated by LPS stimulation in RAW 264.7 cells. Therefore, further study about the biological mechanisms related with the NO inhibitory effect of VLFEP is required.
Taken together, our results suggest that applying fer-mentation, EAE, and an ultra-filtration system improved the isolation of a polysaccharide component from