Wave motion has been suggested as one of the main factors driving morphological variations in species within Laminariales (Gerard and Mann 1979, Druehl and Kemp 1982, Koehl and Alberte 1988, Kraemer and Chapman 1991a, 1991b, Kawamata 2001, Blanchette et al. 2002). However, Miller et al. (2000) suggested for Pelagophycus porra a combination of genetic information and environmental pressures. The ecophysiological features of each species might play a relevant role in the species geographical and vertical distribution (Edwards and Hernandez-Carmona 2005, Edwards and Estes 2006), but most of the studies have been focused on subtidal species / populations and less information is available for intertidal populations (McConnico and Foster 2005).
Morphological variability has been related to responses to the hydrodynamic forces of the environment between exposed and protected areas (Kraemer and Chapman 1991a). In laboratory experiments it has been shown that those morphological differences are related to associated nutrient uptake (Kraemer and Chapman 1991b). This relationship between water motion and morphology has been shown for Eisenia arborea subtidal populations by Roberson and Coyer (2004), who also point out that this variability also has a genetic background, even at very short spatial scales. In a previous study on intertidal populations of the same species, but not related to wave exposure, Parada et al. (2009) observed positive morphological correlations between maximum stipe length and stipe width. Some individuals had short and wide stipes, while others had long and narrow ones.
However, besides water motion role in morphological and size variability in this species it also has been documented the presence of hollow stipes in intertidal populations of E. arborea intertidal in Punta Eugenia (Parada 2005). The same phenomenon was observed in subtidal populations with a geographical north to south tendency in the presence of hollow stipes (Matson and Edwards 2006, 2007). These authors suggested an environmental control of the population morphology. Moreover, slow growth rate and a seasonal reproduction of intertidal populations of this species (Parada et al. 2009) might play an important role to produce unexpected high variability between exposed and protected sites.
Our hypothesis is that morphometric and morphological differences between individuals of E. arborea are related to protected and exposed sites, particularly on intertidal populations of Punta Eugenia. Therefore, our specific goals were 1) to determine the effects of wave exposition in thalli size morphology and 2) to evaluate if those wave exposures might play a role in the presence of hollow thalli, observations that were done also at different seasons.
Influence of water action in an intertidal population of Eisenia arborea was evaluated near the lighthouse (El Faro) in Punta Eugenia (see Parada et al. 2009 for site description). Wave exposure faced north-east on large sedimentary rocks. The protected site was distant at 1 km from the exposed one. Sampling were developed at the lowest tides to include most of the intertidal populations, along 100 m of coast, on each site. To evaluate the effects of wave exposition in thalli morphology a total of 189 individuals were sampled in February, May, July, and August of 2004. During each visit at each site, plant total length, the stipe length, and perimeter (at 3 cm from the base), the length at the dichotomy of the stipe and the number of fronds present were recorded. To evaluate the presence or absence of hollow stipes in the samples from protected and exposed sites we used the samples from May (n = 36), July (n = 49), and August (n = 47) of 2004, by excising a transversal section at 1/3 of the stipe above the holdfast.
Water temperature (Table 1) was obtained by Centro de Investigacion Cientifica y de Educacion Superior de Ensenada (CICESE). We compared the seasons and the sites using the independence χ2 test (α = 0.05) (Zar 1996). Principal component analysis was performed (ACP, α = 0.05) (Zar 1996) to evaluate which variables explain better the morphological variation of the species and this were graphically expressed. In addition, the dataset was tested for normality with Kolmorogov-Smirnov and homoscedasticity with Bartlett tests. Because the assumptions were met we performed an ANOVA one way analysis (Model I) between sites (α = 0.05).
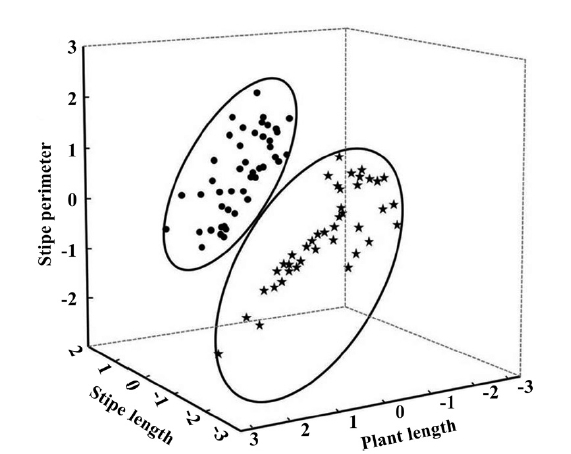
Punta Eugenia intertidal populations of Eisenia arborea is composed by a very dense plant canopy where individuals may reach maximum 2.2 m in total length, stipes can reach 1 m, thalli can have 12 cm in perimeter and frond density was 140 fronds per plant. Principal component analysis indicated that the most significant variables were plant length explaining the 44.3% of the total variance, stipe length 20% and stipe perimeter 14.5%
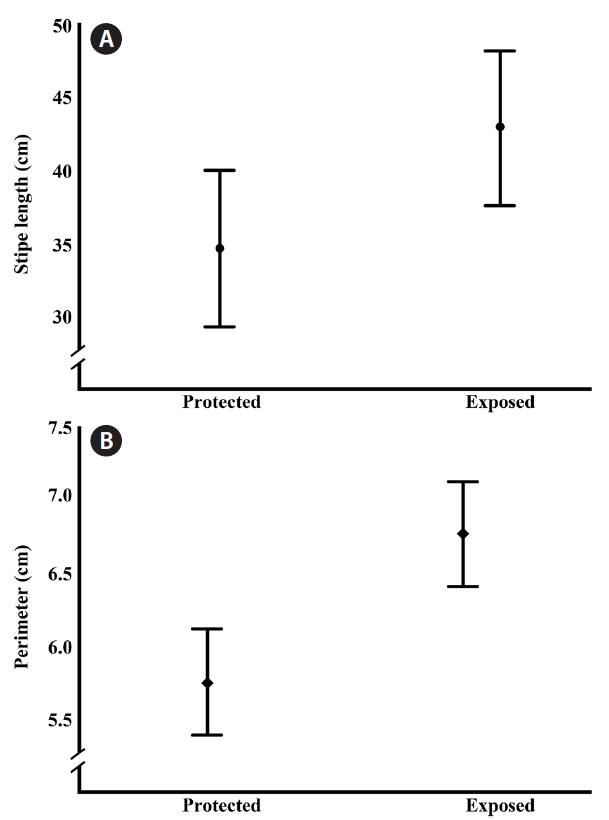
respectively. All of the significant variables participate with the 78.8% of the accumulated variance; we did not find differences in frond density or length of the dichotomy of the stipe. The plot of the 3 significant factors showed 2 groups (Fig. 1) clearly segregating the exposed vs. protected sites. Based on this observation a one way ANOVA was performed where we found significant differences in the stipe length (F(1,83) = 4.716, p = 0.033) and stipe perimeter (F(1,83) = 15.579, p < 0.001) (Fig. 2). The average in stipe length was 42.9 cm for the exposed sites and 34.6 cm for the protected ones (Fig. 2A). In the case of the average in stipe perimeter was 6.8 cm for the exposed sites and 5.8 cm for the protected sites (Fig. 2B).
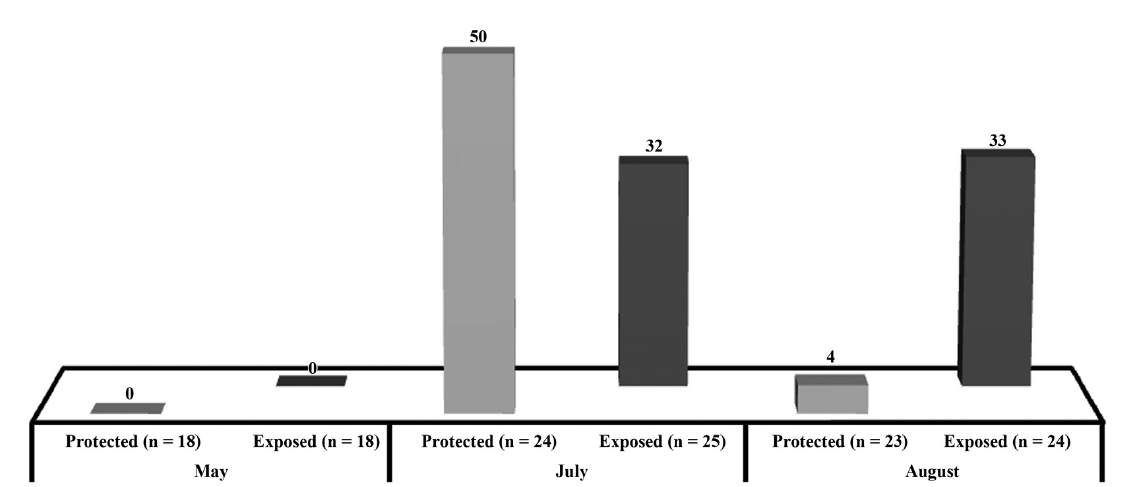
Respect to the presence of hollow stipes, we found in May 0% of plants with hollow stipes but in July this presence increased to 50% in the protected sites and 32% in the exposed ones, to finally decrease to 4% in the protected sites remaining at 33% in the exposed sites (Fig. 3). The independence test did not show any significant difference in the protected (χ2 (α = 0.05, v = 2) = 5.991; χ2 = 21.52; p < 0.05) or exposed site (χ2 (α = 0.05, v = 2) = 5.991; χ2 = 7.73; p < 0.05).
Our results have shown that this intertidal populations of E. arborea do compensate morphologically to environmental stress. This was clearly proved in the stipe morphology as we found differences in length and diameter. Similarly, kelps show important phenotypic plasticity in response to environmental heterogeneity, such as wave-exposure in Ecklonia radiata (Wernberg et al. 2003, Wing et al. 2007), or local environment as in Macrocystis pyrif
era (Demes et al. 2009). Also morphological change has been documented in Laminaria setchellii (Klinger and De Wreede 1988) and other kelps species (Gerard and Mann 1979, Koehl 1986). The relevance of this morphological change might be linked with higher survivorship. Parada et al. (2009) have shown that Eisenia arborea population at Punta Eugenia has a slow growth rate but a higher survivorship. This phenomenon might be related to a morphological adaptation, with longer stipes and more robust ones in exposed areas. Roberson and Coyer (2004) also found morphological variations in subtidal populations, but they suggested that genetic variability was also present in the morphotypes. Tellier et al. (2009) using a combination of four genetic makers, located in the three genomic compartments (chloroplast, mitochondria, and nucleus), showed the presence of two highly divergent lineages in Lessonia nigrescens, a South Pacific intertidal kelp, suggesting even the presence of cryptic species (similar morphology and thus considered as only one taxonomic species). They also showed complete reproductive segregation and different ecological niches, in sporophytes but also in microscopic gametophytes. This
is also an hypothesis to test in E. arborea, considering the geographical distribution of the species, submitted to different oceanographic regimes and also its vertical distribution. Intertidal populations have not been described South of southern Baja California. Matson and Edwards (2006) have shown geographical trends in the presence of hollow stipes in Eisenia arborea. Moreover, our results show a temporal component in relation to summer conditions (high temperature and low nutrient concentration in seawater). South of 30° N in the California current there is pattern of turbulence according to north surface winds favoring cold upwelling’s and waters rich in nutrient. This happens from May to June, coincident with the presence of hollow stipes. A second upwelling occurs in autumn with a strong water stratification from August to November (Cervantes-Duarte et al. 1993). Although May presents upwelling conditions no hollow stipes were observed. A similar situation was expected for August, but hollow stipes were present in half of the sampled individuals. On the contrary, in July we observed the highest frequency of hollow stipes, a season of no described upwelling. Moreover, there are differences in the proportion of hollow stipe respect to protected vs. exposed areas. The influence of warmer waters is involved in the process and no relationship with upwelling (Cervantes-Duarte et al. 1993, Matson and Edwards 2007) but also the fact that plants grow more in spring (Parada et al. 2009) might influence the presence of hollow structures. However a physiological trade-off can be suggested for stress due to high temperatures and low nutrients. Medullar tissues can play the role of nutrient reserves, which are exhausted in stressing conditions. It is known that spore quantity depends on tissue nitrogen content (Gerard 1997), which is also inversely correlated to water temperatures (Zimmerman and Kremer 1984, 1986). On the other hand, Hernandez-Carmona et al. (2001) demonstrated that E. arborea, differing from M. pyrifera, maintained high tissue nitrogen content. This phenomenon would help to maintain its reproductive structures during the stressing period.
Mortality are low in the growth season and it seems that there is no relationship with the presence of hollow stipes or any other mechanical advantage / disadvantage for the plants (Matson and Edwards 2007).
The influence of water motion in plant development is not easy to evaluate because the difficulties that represents the isolation of the environmental factors (Hurd 2000). But, the continuous movement related to the waves might select individuals more ‘elastic’ with this tubular morphology with a perimeter of 5.2 cm in stipe (diameter ? 1.65 cm) representing plants with large sizes. If we evaluate this size in relation to the growth rate of the plant (0.2 mm per day) we might estimate that plants with hollow stipes represent individuals in their second year of life, but more experiments are needed to determine age and sexual maturity. Transplant experiments would be needed to evaluate if hollow stipes can be induced in individuals from geographically separated populations. Such transplants have been already performed to test heavy metal tolerance in the intertidal kelp Lessonia nigrescens on the north Chilean Pacific coasts (Correa et al. 2006). The suggested evaluation of genetic differences for E. arborea even at short spatial scales could be useful not only for taxonomical reasons but also for stock management strategies in harvested populations in the cost of the North-east Pacific.