The re-examination of morphological and anatomical characters of Laminaria appressirhiza and L. inclinatorhiza collected from different localities in the Sea of Okhotsk was performed. Despite their commercial and ecological importance to the region they have not been comprehensively reviewed since their first description in 1970. Our results show that some original diagnostic key characters (e.g., shape of holdfast, shape of sporangial sori, and dissection of blade) are not stable and have deviations from the type morphology when plants grow in different environments. In L. inclinatorhiza, the sporangial sori development occurred differently to the pattern indicated in original species description as they did not develop simultaneously on both sides of the blade. Instead, the sporangial sori outlines on both sides of the blade did not coincide at first and only became coincident later. Also, a deep-water population of L. inclinatorhiza with an unusual and interesting morphology, growing at depths of 15-25 m on opened rocky coasts in Taujskaya Bay (northern part of the Sea of Okhotsk) was found. The stable diagnostic key characters to distinguish these two species are the cone-like, multilayered, very thick and massive holdfast (in L. inclinatorhiza) and rolled margins of blades, lamellar rosette-like part of thallus, and sporangial sori developing only on one side of the blade (in L. appressirhiza). The ecological characteristics, distribution, and abundance of both species in the Sea of Okhotsk are discussed. Both species are perennial and widely distributed in the region. L. appressirhiza is more often found as a subdominant species among other kelps, forming maximum biomass and density of 7-9 kg and 8-25 plants per 1 m2, respectively. L. inclinatorhiza sometimes forms local mono-species communities with maximum biomass and density of 10-12 kg and 10-15 plants per 1 m2, respectively.
Kelp forests are the most important components of the ecosystems of temperate and polar rocky shores. They ensure high productivity, biodiversity, and healthy ecological condition of the marine biota and they constitute habitat and breeding grounds for a large diversity of marine life forms (Klochkova et al. 2009, Belij 2011, Zambounis et al. 2012). Regarding the diversity and natural stocks of various marine organisms, including the macroalgae, the Russian Far East represents one of the richest regions of the world. However, due to geographical and historical reasons it remains one of the least studied areas. The extensive coastline of the Russian Far Eastern region is mostly unpopulated and inaccessible, which makes field collections very difficult, one example being the northern areas of the Sea of Okhotsk. Recently, a renewed interest in the macroalgal ecosystems in this region has arisen (e.g., Klochkova et al. 2010, 2012) because of the economic development and fishery demands in the area (Belij 2011). In this region, the laminariaceaen algae are dominant or co-dominant members of macrophytobenthic communities in the littoral and sub-littoral zones. Three digitate species of
The species
>
Sample collection and specimen observation
Type specimens of
During the course of this study, we observed numerous freshly collected specimens of
Specimens of
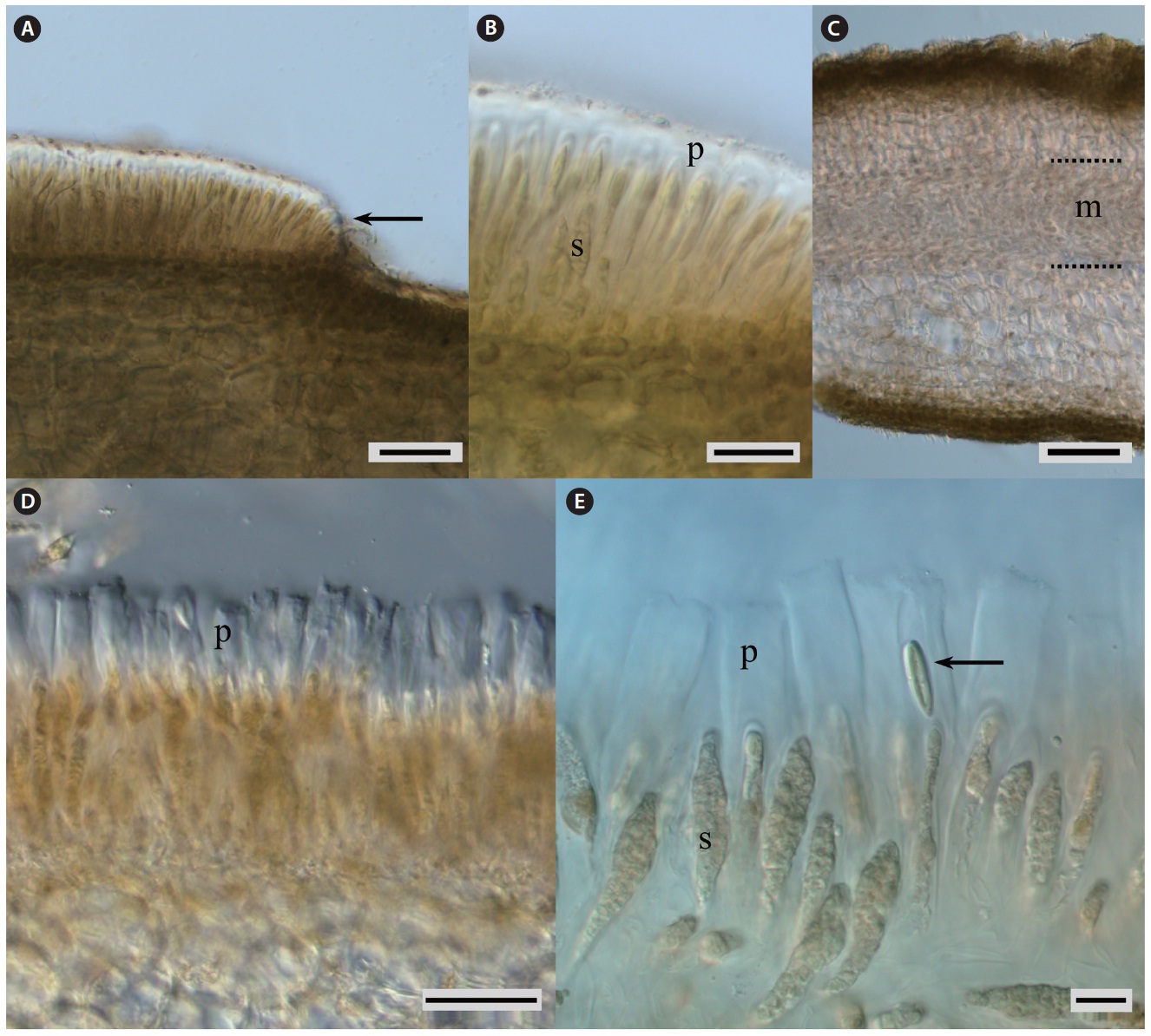
For microscopic observations, a dry piece of thallus was re-hydrated in sterilized seawater, cut with a fine razor blade, and observed under a microscope. Micrographs were taken with Olympus DP50 digital camera (Olympus, Tokyo, Japan) affixed to an Olympus BX50 microscope using Viewfinder Lite and Studio Lite computer programs.

Specimens used for the demonstrations of the distinctive morphologies of species in present study, their collection site and date
>
Original diagnosis of Laminaria appressirhiza by Petrov and Vozzhinskaja (1970)
The blade is roundish or oval, dissected, up to 500-1,000 mm long. Freshly-collected blade is rolled into a tube; in nature often looking like a rosette with widely spreading fronds. Stipe is erect, about 10 mm in diameter and 150-280 mm long; in plants collected from littoral zone stipe can be up to 40 mm long. Holdfast originate from the stipe at almost right angle; haptera are rigid, branch multiple times (at each 10 mm), becoming thinner toward the tips. In cross-section, the medullar part of the blade occupies from 17 to 40-60% of its thickness. Mucilage ducts are quite large, 40-56 × 80-100 μm, located at a distance of 250-350 μm from each other. The distance from the outer surface of blade to the center of mucilage duct and then to the blade’s medullar layer is 1 : 1.5-1 : 2.5. Mucilage ducts are absent from the stipe and haptera. Sporangia develop in August-September only on one side of the blade, facing outward. Thus, the herbarium specimens have rolled margins of blades in places of sporangial sori localization.
Type (Fig. 1A & B). Sea of Okhotsk, Gizhinskaya Bay, Udachi Inlet, depth 8 m, Aug 28, 1964, collected by V. B. Vozzhinskaja. Kept in Botanical Institute of Russian Acad. Sci., Leningrad (LE, St. Petersburg).
>
Original diagnosis of Laminaria inclinatorhiza by Petrov and Vozzhinskaja (1970)
Blade is roundish, elongated-elliptical or ovate, dissected or non-dissected (in wave-sheltered areas near Shantar Islands), flattened or concave at its base, up to 740 mm long. Stipe is erect, about 10 mm in diameter and 30-70 mm long. Haptera are loosely branched; holdfast forms a big cone, which is almost as long as the stipe. Holdfast originate at an angle from the stipe and haptera begin to branch only in the lower portion; often they do not branch for the first 40 mm from the stipe. Haptera reach maximum width (5.5-6 mm) and thickness (up to 4.2 mm) in the middle part, but they are narrower (up to 2.7 mm) where they originate from the stipe. Whole holdfast can be easily broken from the stipe, especially in herbarium specimens. In cross-section, the medullar part of blade occupies about 22% of its thickness. In the blade, the mucilage ducts are ca. 80-120 μm in size, located at a distance of 20-180 μm from each other. The distance from the outer surface of blade to the center of mucilage duct and then to the blade’s medullar layer is 1 : 2.1-1 : 2.5. In stipe and haptera, mucilage ducts are absent. Sporangia develop in late July-September on both sides of the blade, the sporangial outlines coincide in shape.
Type (Fig. 1C & D). Sea of Okhotsk, Kavacha Bay (northwest Kamchatka), depth 12 m, Aug 24, 1963, collected by V. B. Vozzhinskaja. Kept in Botanical Institute of Russian Acad. Sci., Leningrad (LE, St. Petersburg).
>
Observations of morphology by present authors
The following descriptions are based on our personal observations of collected samples. Morphologically,
Laminaria appressirhiza
The stipe is elastic, cartilaginous, cylindrical, 15-30 cm long, ca. 1-1.5 cm in diameter, olive, yellow-brown or dark-brown in color, darker in its upper part (Fig. 2C-E). Haptera (Fig. 2A & C) are rigid, cartilaginous, relatively thick, dichotomously or irregularly dichotomously branched, slightly broadening where they branch and becoming thinner to the tips. Young holdfast is light yellow-brown or olive in color, whereas old holdfast is dark-brown or black-brown. In cross-section, the color of the medullar layer is not different from the color of the next layer in both young (Fig. 2D) and old plants (Fig. 2E). Holdfast most often originates from the stipes at almost right angle. In plants growing on cobble, it originates from the stipes at an acute angle.
The blade is roundish or oval in shape (Fig. 3C & D), up to 1 m in length and ca. 1 m in width or even wider (Fig. 2A & B), dark-olive, brown or reddish-brown in color, without bullation at any age. In cross-section, the young blade is ca. 430 μm thick; the medullar part occupies from 15 to 18% of its thickness. In fertile plants, the blade is ca. 600 μm thick and the medullar part occupies ca. 27.5% of its thickness (Fig. 6C). At the beginning of linear growth, both young and older plants have an unsplit and more or less concave blade. During subsequent growth, the blade becomes more concave, thus resulting in formation of its funneled basis. Then the longitudinal splits occur. The number of straplike blades vary with the amount of exposure to waves and the lacerations extend almost to the base of the blade. Sometimes, the lacerations are so deep that even the stipe splits. Old blades can have 15-30 straps of 3-5 cm at width. Thus the lamellar part of thallus often looks like a rosette with straplike blades spreading radi-
ally. Sporangial sori develop more often on one side of the blade in its lower part (Fig. 3A) in late July-September. In partially and completely dried adult plants, the margins of straps are almost always rolled into a tube, even when the plants are not fertile (Fig. 2B). In young plants not bearing sporangial sori, the mucilage ducts are smaller (40-50 μm in size) and appear on both sides of the blade; however, they are more pronounced on one side of the blade than on the another. In some blades, the network of mucilage ducts beneath the cortex layer could be seen even from the outer surface (Fig. 3B). In fertile plants, the mucilage ducts are large (up to 109 μm in size) and appear only on one side of the blade that does not have sporangial sori. Mature unilocular sporangia are ca. 51 μm in length (Fig. 6A). Paraphyses are 77-96 μm long, narrower at the distal end (Fig. 6B).
Specimens observed in present study. Continental coast of the Sea of Okhotsk, Taujskaya Bay, collected by N. G. Klochkova and M. N. Belij on Jul 14-15, 2008 (vouchers CH2208, CH2209, CH2210). Western Kamchatka, Ust- Hajryuzovo village and Ptichij Island, collected by N. G. Klochkova and A. A. Emelyanova on Jul 25-26, 2004 (specimens illustrated by Klochkova et al. 2009).
Laminaria inclinatorhiza
During the present study we observed that the key characters listed by Petrov and Vozzhinskaja (1970) do not always precisely apply to the morphology of
Specimens observed in present study. Continental coast of the Sea of Okhotsk, Taujskaya Bay, collected by N. G. Klochkova and M. N. Belij on Jul 14-15, 2008. Western Kamchatka, Ust-Hajryuzovo village and Ptichij Island, collected by N. G. Klochkova and A. A. Emelyanova on Jul 25-26, 2004 (specimens illustrated by Klochkova et al. 2009).
>
Description of typical morphology of Laminaria inclinatorhiza (voucher CH2207)
During our field studies, we often collected plants with morphology fitting the description and illustrations of Petrov and Vozzhinskaja (1970) from the shallow wave-swept waters (Fig. 4A & B). Young blades can be entire, roundish or elongate-elliptical in shape. In younger plants, the blades are slightly concave, resembling a hood; however they later tend to become broader and roundish. In older plants the blades are up to 1 m in length, very dissected, having 2-3 or up to 10-12 straplike blades in younger and older thalli, respectively, each being 1 to 5-8 cm in width. The lacerations extend almost to the base of the blade. The blades are quite fragile and easily detachable from the stipe, thus we often found holdfasts without blades on the substrate (Fig. 4A & I). Bullation is not observed at any age and the blade’s margins are never rolled such as in
Mucilage ducts or canals were not found in our samples. In cross-section, the juvenile blade is ca. 141-192 μm thick; medullar part occupies ca. 16.6-23% of its thickness. In fertile plants, the blade is ca. 1,650 μm thick and the medullar part occupies ca. 13% of its thickness. Sporangial sori develop in late July-September on both sides of the blade in its lower and middle parts as wide shapeless spots with not coincident outlines at first. Upon further development, the spots outlines more or less coincide. Mature unilocular sporangia are 51-58 μm in length (Fig. 6D). Paraphyses are ca. 115 μm long, narrower at the distal end (Fig. 6E).
We found an unusual and interesting deep-water population of
In this population of
& 5E-G). The holdfast is tapered, shaped like a cone in cross-section. A characteristic ring of small hapteral outgrowths of bright lilac-violet color develop at the top of the holdfast (Figs 4G & 5F). With further growth, the bright lilac-violet color gradually turns into red-violet. In young plants, the stipe is violet in color (Fig. 4G). In old blades, the stipe is cylindrical, thick, 1.5-1.7 cm in diameter and 20-25 cm long. Under the sea and for a short time after removal from seawater, the stipe has a characteristic white-violet or lilac color and is slightly fluorescent. Upon drying, it becomes red-violet in color and the fluorescence disappears. The upper part of stipe is flattened, slightly broadened, light or yellow-pink colored, which is very different from the coloration of the lower 2/3 of stipe and the blade. The blades have no bullation at any age. Mucilage ducts or canals were not found in our samples, including young and older thalli.
Sporangial sori start to develop on both sides of blade in its lower and middle parts, forming shapeless spots that do not coincide in outline at first (Fig. 5A-D). Upon further development, the outlines of the spots more or less coincide and the sporangial tissue spreads to the blade’s middle part. The juvenile 15 cm-long plants (i.e., at early stages of development during 1st year of growth) also had sporangial tissue (Fig. 4F). We consider that the most abundant sporangial development probably occurs from late August to early September.
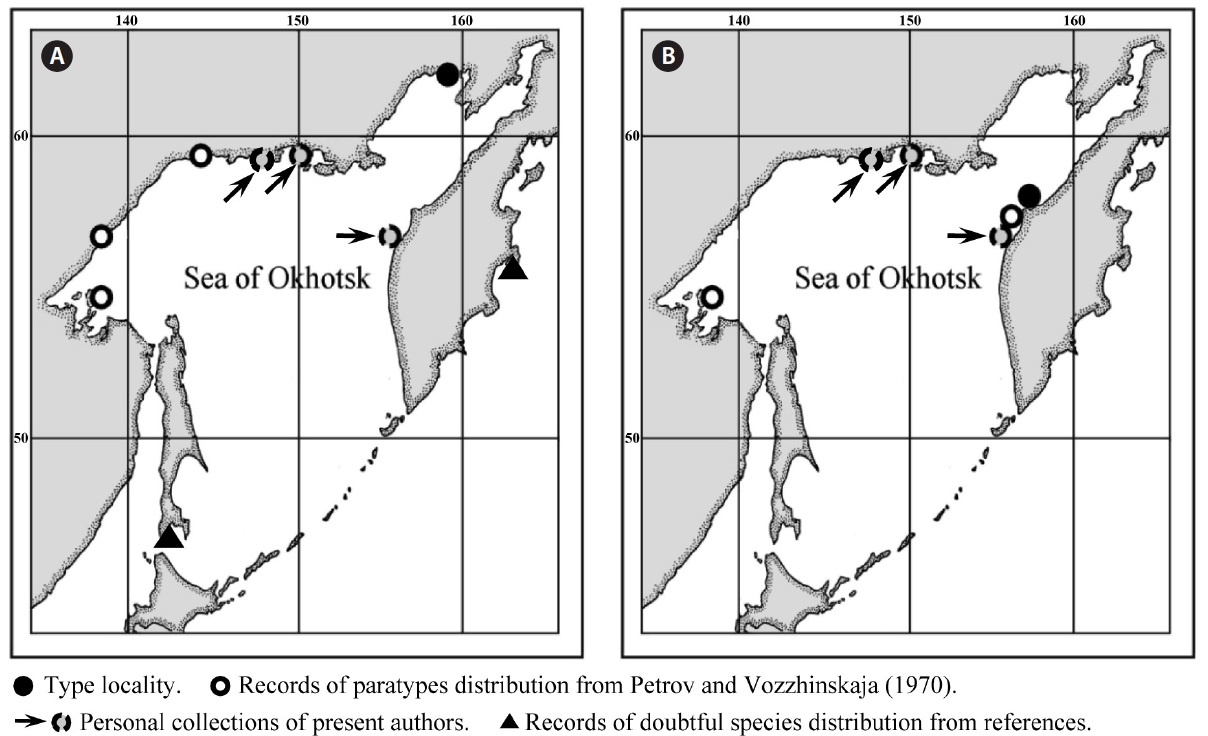
The currently known distribution of
Our results support the existence of two digitate
The unique macroalgal flora of the Sea of Okhotsk is due to the system of currents in the region, mosaic climatic and hydrological conditions in its different areas, and geological history, which resulted in long-term geographic isolation (Klochkova et al. 2012). To date, five brown and five red algae have been reported as endemic species of this region; the brown algae
The Sea of Okhotsk and Arctic Russia were named as regions of the world still not comprehensively studied, where systematic studies remain morphologically based and there has been little recent work (Bolton 2010), i.e., phylogenetically based systematics. However, it should be emphasized that for many algal taxa distributed in these regions, including endemics and commercially important kelp species, information on morphology and anatomy and differences in the morphogenesis of species and their forms is very limited. These basic studies are essential because incomplete understanding of genus-or species-specific (or species form-specific) morphology and anatomy can lead to a wrongful presumption of the existence or non-existence of a taxon. For example,
In this study, we focused on morphology, anatomy, ecology, distribution and abundance of two endemic species from the Sea of Okhotsk,
Some characteristics of
In species diagnosis of
According to our observations and differently to what was said by Petrov and Vozzhinskaja (1970), in the plants of
The shape of sporangial sori is not a stable morphological character and can deviate from the norm in different populations of the same species according to growth environmental conditions (e.g., Korolyova 2004, Saushkina 2006). Saushkina (2006) studied changes in the reproductive strategies of laminariaceaen algae in Kamchatka under unfavorable environmental conditions and reported that even <1℃ difference in seawater temperature can significantly influence monthly seasonality and speed of spore formation. In the areas experiencing high levels of anthropogenic pollution, sporogenesis deviates from the norm. It should be noted, however, that Taujskaya Bay in the Sea of Okhotsk is a clean area, thus sporogenesis in the juvenile plants of
In conclusion, our new data extend the knowledge on two commercially important laminariaceaen algae from the Sea of Okhotsk and highlight the need for further studies on the biology of development and reproduction of other macroalgal species in this area.