Undaria pinnatifida (Harvey) Suringar, called “miyok” in Korean, is a brown alga and a food source for marine animals and humans and is also used as a nursery environment for many marine organisms (Yamanaka and Akiyama 1993, Sohn 1998). In general, the alga is divided into southern and northern types based on morphological features (Okamura 1915, Taniguchi et al. 1981, Stuart et al. 1999). The southern type, U. pinnatifida f. typica Yendo, has a short stipe, shallow pinnate division of the blade, and sporophyll development that is often confluent with the base of the blade. The northern type, U. pinnatifida f. distans Miyabe & Okamura, is characterized by an elongated stipe and a deeply divided blade, with sporophyll development confined to the basal portion of the stipe (Kito et al. 1981, Akiyama and Kurogi 1982, Ohno and Matsuoka 1993). Morphological characters used to distinguish U. pinnatifida f. distans from f. typica are midrib width, stipe length, the degree of pinnate division, and sporophyll position on the stipe (Kito et al. 1981, Taniguchi et al. 1981).
It is well known that the morphological features of the two types of U. pinnatifida are related to environmental factors (Saito 1960, Taniguchi et al. 1981, Sohn 1984). Saito (1962) reported that stipe length and plant length are stable morphological features, irrespective of environmental conditions. Additionally, the transplanted descendants of the two U. pinnatipida types grown in the same environmental conditions remain distinguishable by pinnule length, incision depth, midrib width, plant length, and plant weight (Kito et al. 1981, Hara and Akiyama 1985, Lee and Sohn 1993). The sporophytes of the two Undaria types also differ in morphological features and blade color (dark brown in the northern type; light brown in the southern type) at similar ages and are 20-22 cm long (Hay and Villouta 1993, Castric-Fey et al. 1999). The two Undaria types also differ in the physiology of the gametophyte and sporophyte stages (Akiyama 1965, Kim and Nam 1997, Castric-Fey et al. 1999).
U. pinnatifida is an important nutrient source for diet and food additives because of their high content of minerals, trace elements, proteins, lipids, and carbohydrates (Rhee 1972, Hong et al. 1991, Lee 2004, Manivannan et al. 2008). Nutritional content varies with species, geographical location, season and temperature in seaweeds (Solimabi et al. 1980, Dawes et al. 1993, Kaehler and Kennish 1996). In U. pinnatifida, the proximate composition, alginic acid, and mineral content are different among the plant parts such as the frond, stipe, and sporophyll (Rhee 1972, Lee 2004). Thus, we expect that the proximate, mineral, amino acid, and fatty acid compositions will be different among U. pinnatifida populations grown under different environmental conditions. The nutritional value of edible U. pinnatifida is very beneficial for pregnant woman, adolescents, and elderly, which are all exposed to a risk for nutritional deficiency such as fatty acids and calcium.
Morphological differences are also found within the U. pinnatifida f. distans populations cultivated in Korea (Sohn 1984). The sporophytes of U. pinnatifida f. distans grow well in wave-exposed areas, as do their natural populations. Cultivation of U. pinnatifida was initially attempted in Kijang, with Kijang miyok now obtaining the highest price among cultivated U. pinnatifida products in Korea. Additionally, natural U. pinnatifida f. distans
sporophytes from Wando and Jindo are well known because for their high quality texture and nutrition (Im et al. 2006). The harvesting periods of native and cultivated U. pinnatifida sporophytes are generally between March and early April, but that of the Jindo population is from the end of July to early August. Thus, the Kijang, Wando, and Jindo U. pinnatifida populations are representative northern type populations in Korea. The aims of this study were to test whether the three U. pinnatifida f. distans populations could be characterized by morphological features and biochemical composition and to compare phenotypic variations among the populations based on environmental conditions.
Sporophytes of U. pinnatifida were collected from a cultivation area near Kijang on the eastern coast of Korea in March 2006. U. pinnatifida sporophytes were collected from the rocky shores of Wando (a natural habitat) in April 2006 and from Jindo in July 2006 (Fig. 1). The collection time differed based on harvesting periods for these areas, but they were all at the same developed sporophyll stage. The Undaria sporophytes sampled at Chungdeungdo, Jindo, Korea were different in morphology (Fig. 2) compared to that of the Wando and Kijang plants, which have the morphological features described in Fig. 3. Morphological characters including plant and sporophyll weights were measured in 25 plants selected randomly from each population (Fig. 3). Plant weight including the sporophyll was estimated, and then the sporophylls were cut for weighing. The midrib thickness of U. pinnatifida was measured with a digital Vernier caliper (Mitutoyo, Tokyo, Japan).
A one-way analysis of variance was applied to compare
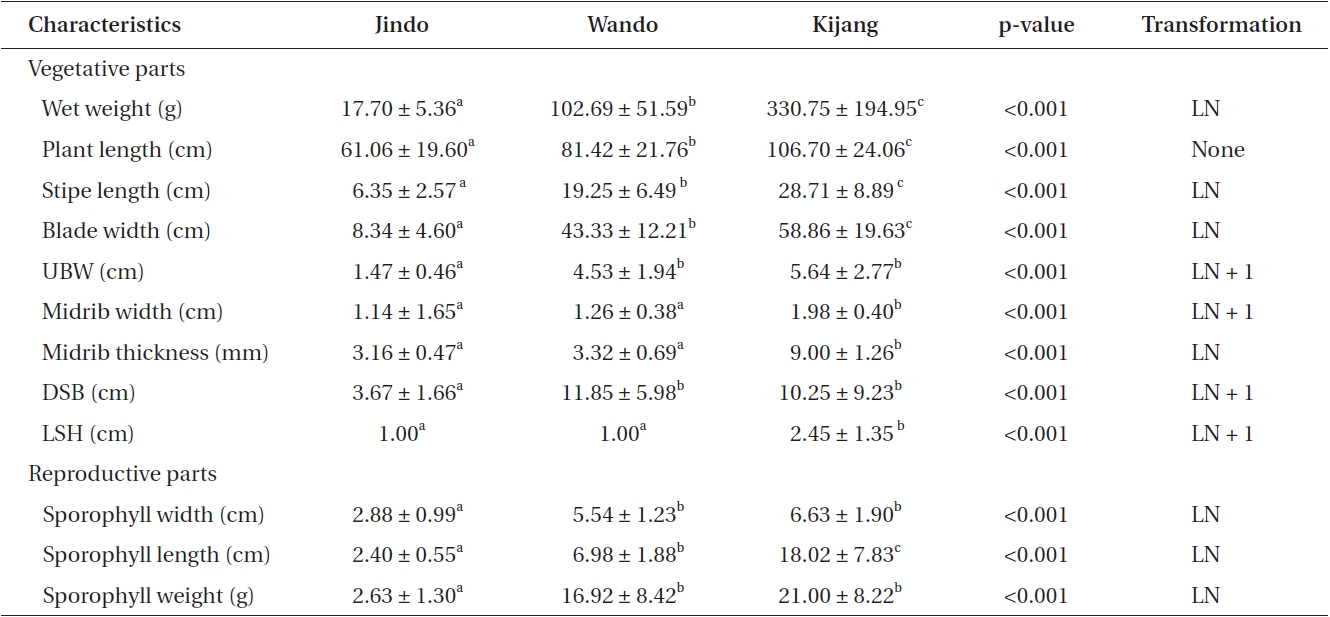
[Fig. 3.] Undaria pinnatifida morphometric measurements. PL, plant length; FL, frond length; BW, blade width; UBW, undivided blade width; MW, midrib width; SL, stipe length; SPW, sporophyll width; SPL, sporophyll length; DSB, distance between sporophyll and blade; LSH, length between sporophyll and holdfast.
morphological parameters among the three Undaria populations, including plant and sporophyll weights. Prior to analysis, homogeneity of variance was tested using Cochran’s test, and data were transformed when necessary (Underwood 1997). Tukey’s multiple comparison test was applied when significant differences between means were detected (p < 0.05) (Sokal and Rohlf 1995). Statistical analyses were carried out using STATISTICA version 5.0 (Statsoft, Tulsa, OK, USA).
Moisture content of U. pinnatifida samples was determined after drying in an oven at 105℃ until a constant weight was obtained, and ash content was estimated after overnight heating at 550℃. Crude lipid was extracted in a Soxhlet extractor (SX-6; Raypa Co., Catalonia, Spain), and protein content was examined with an automatic Kjeldahl analyzer (B-339; Buchi Co., Flawil, Switzerland). Fiber content was analyzed by the method of Henneberg Stohmann (Minowa et al. 1995). Carbohydrate was calculated by the following equation (Dubois et al. 1956): % carbohydrate = 100 - (moisture + crude ash + crude lipid + crude protein + crude fiber). Mineral analyses were carried out on samples digested with nitric acid. Major mineral elements (Ca, Mg, K, and Na) and trace elements (Fe, Cu, and Zn) were measured by atomic absorption spectrophotometry (Solaar-M5; Thermo Elemental Co., East Anglia, England). Total phosphorus was measured using a spectrophotometer (UV- 1601; Shimadzu Co., Tokyo, Japan) according to the method of Topping (1972). Samples for amino acid analysis were hydrolyzed in 15 mL of 6 N HCl at 110℃ for 24 h in a dry oven. The samples were subsequently evaporated using a vacuum rotary evaporator, filtered (Ø, 0.2 μm), and then amino acids were determined with an amino acid analyzer (S433; Sykam, Eresing, Germany). Approximately 0.2 g of extracted lipid was methylated with 5 mL of 0.5 N methanolic NaOH solution followed by heating for 2 min and extracted with n-heptane. The methyl esters were quantified by a gas chromatograph (6890N; Agilent Technology, Palo Alto, CA, USA) equipped with a flame ionization detector and a SP-2560 column (100 m × 0.25 mm; film thickness, 0.20 μm). The initial temperature of column was 140℃ for 5 min, and temperature was programmed to increase by 4℃ min-1 to 240℃. The final temperature was held for 19 min. Injector and detector temperatures were set at 260℃, the carrier gas was nitrogen (N2), and flow rate was 0.8 mL min-1. Each fatty acid was identified by comparing its retention time with that of a methyl ester mixture (Sigma Chemical Co., St. Louis, MO, USA). Fatty acid contents were calculated using peak area percentages of total fatty acid content.
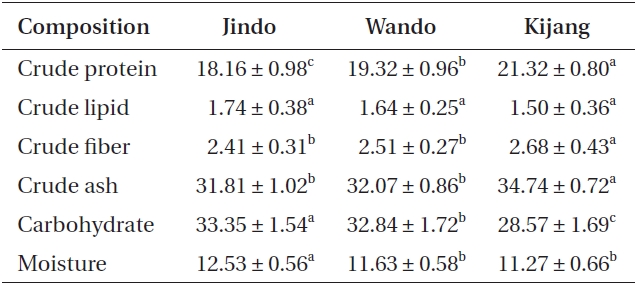
Sporophyte weights of U. pinnatifida at the three locations were 17.70-330.75 g wet weight, and plant lengths were 61.06-106.70 cm. Plant weights and lengths were significantly different among the three populations (Table 1). U. pinnatifida sporophytes growing on cultivation ropes at Kijang were two times longer and 19 times heavier than those of sporophytes of the native Jindo population. Average stipe lengths of U. pinnatifida sporophytes were 6.35-28.71 cm, and differences in stipe length were also found among the three populations (Table 1). Blade width of Undaria sporophytes was seven times greater for cultivated plants in Kijang than those collected at Jindo. The undivided blade width of the Jindo population was significantly smaller than that of Wando and Kijang plants. Additionally, average midrib widths and thicknesses of sporophytes were quite similar between native U. pinnatifida populations but were significantly greater in Kijang sporophytes. The distance between the sporophylls and blade (DSB) was 3.67-11.85 cm, and it was significantly greater in the Wando and Kijang populations. The DSB of the Jindo population was significantly smaller than that of the other two populations. Sporo-
phyll position on the stipe was different among all three populations. The sporophylls of native populations were positioned just above the holdfast. Length between the sporophyll and holdfast was quite similar among the native populations, but was significantly greater at Kijang than that of the other two populations (Table 1).
The sporophyll features of the three U. pinnatifida sporophyte populations were different. Sporophyll width was largest at Kijang (6.63 cm) and smallest (2.88 cm) at Jindo. Sporophyll width of the Kijang population was two-fold greater than that of Jindo (Table 1). A Tukey’s test revealed that thd sporophyll width of the Jindo population was significantly smaller than that of the other two populations (Table 1). Sporophyll lengths of U. pinnatifida were 2.40 cm at Jindo, 6.98 cm at Wando, and 18.02 cm at Kijang. Average sporophyll length was seven times greater at Kijang than that at Jindo (Table 1). Sporophyll weights of U. pinnatifida sporophytes were 2.63-21.00 g wet weight, which was seven times less at Jindo compared to that in the Kijang population. Thus, Jindo sporophylls were smaller in length, width, and weight than those at Wando and Kijang.
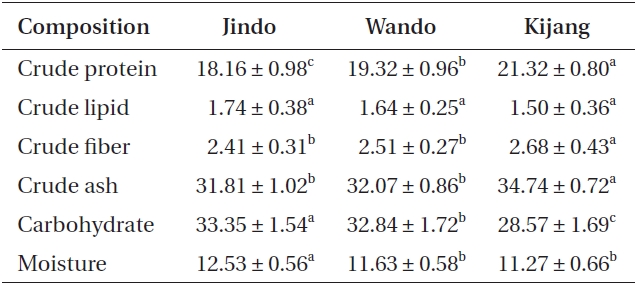
Proximate compositions of Undaria pinnatifida populations were 18.16-21.32% for protein, 28.57-33.35% for carbohydrates, and 1.50-1.74% for lipids (Table 2). Crude protein, fiber, and ash contents of U. pinnatifida were greater in the Kijang samples (21.32, 2.68, and 34.74%, respectively) than those of the Wando and Jindo samples but lipids were not different among the three U. pinnatifida samples. Carbohydrate and moisture contents of U. pinnatifida were 28.57-33.35 and 11.27-12.53%, respectively, and were significantly highest in the Jindo samples.
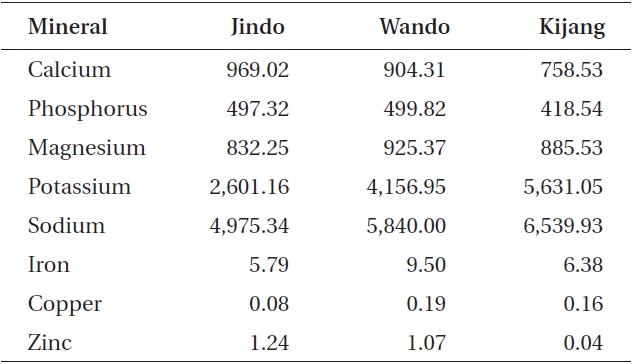
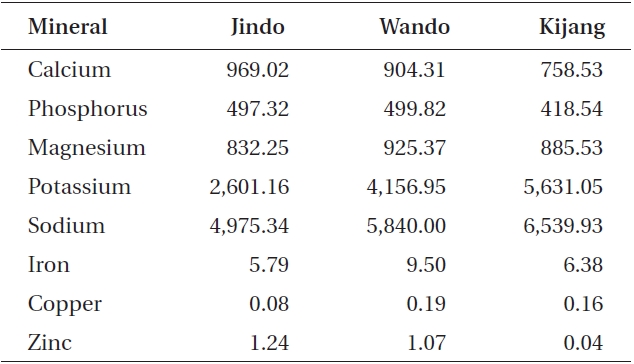
Undaria pinnatifida was rich in calcium, phosphorus, magnesium, potassium, and sodium (Table 3). Calcium and zinc content in the Jindo samples was 969.02 and 1.24 mg 100 g-1 dry weight, respectively, which was the highest among the three Undaria samples. In particular, zinc content of Jindo plants was 30 times greater than that of Kijang plants. Sodium content in the Kijang sam-
ple exhibited the greatest value. The Wando sample had the highest mineral content such as phosphorus, magnesium, iron, and copper (499.82, 925.37, 9.50, and 0.19 mg 100 g-1 DW, respectively).
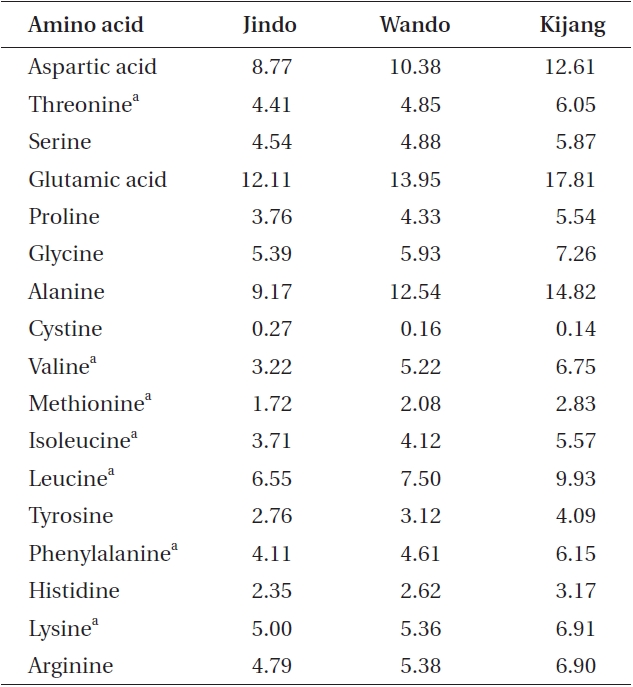
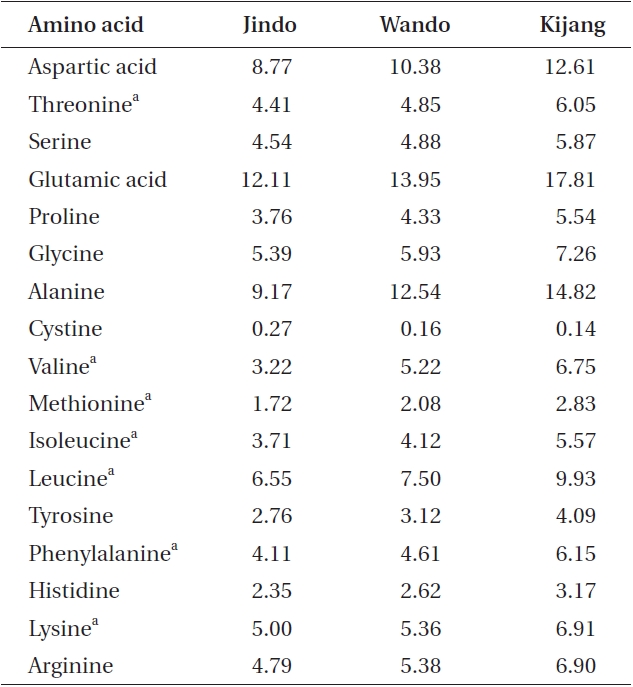
All U. pinnatifida plants collected from the three different rocky shores had seven essential amino acids (Table 4), including threonine, valine, methionine, isoleucine, leucine, phenylalanine, and lysine. Except for cystine, all amino acids exhibited their highest levels in the Kijang samples and the lowest levels in the Jindo samples. Additionally, the amount of essential and total amino acids was maximal in the Kijang sample (44.19 and 122.40 mg g-1 DW, respectively) and minimal in the Jindo sample (28.72 and 82.63 mg g-1 DW, respectively). These results were in agreement with the patterns of crude protein content in the three U. pinnatifida populations.
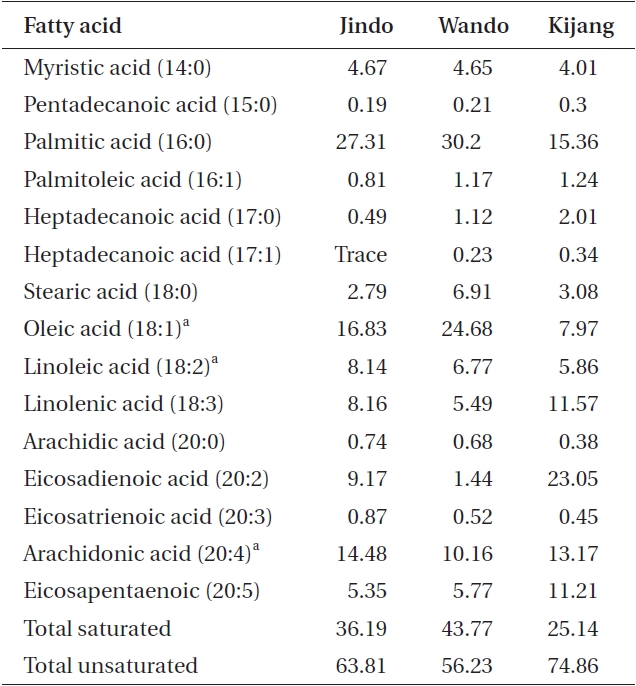
Plants collected from the three U. pinnatifida populations were rich in saturated fatty acids such as palmitic acid, C16:0 (15.36-30.20%), and unsaturated fatty acids such as oleic acid, C18:1 (7.97-24.68%), and arachidonic acid, C20:4 (10.16-14.48%). The proportion of essential fatty acids was highest in the Wando sample (41.61%) followed by Jindo (39.45%), and then the Kijang sample at 27.00%. In contrast, the proportion of total unsaturated fatty acids was the greatest in the Kijang sample (74.86%)
and the least in the Jindo population (Table 5). Additionally, eicosapentaenoic acid (EPA), an n-3 polyunsaturated fatty acid, was two times greater in Kijang plants than that in the Jindo and Wando plants.
Morphological features of U. pinnatifida f. distans sporophytes and sporophylls were significantly different among the Kijang, Wando, and Jindo populations. In particular, plant length and weight of the cultivated U. pinnatifida sporophytes at Kijang were two times longer (106 cm) and 19 times heavier (330 g) than the sporophytes of
the Jindo population (61 cm and 18 g, respectively). Choi et al. (2007) reported that mean plant lengths and weights of U. pinnatifida sporophytes grown in a Busan cultivation farm were 109 cm and 372 g in March 1996, respectively. Sporophyll weight was seven times lighter at Jindo (3 g wet weight) compared to that in the Kijang population (21 g). Jindo sporophylls were also eight times smaller in length than those at Kijang. These results show that U. pinnatifida sporophytes have different morphological features among the three populations, which may be related to the environmental conditions of their habitats.
Kijang Undaria plants showed the highest total protein, crude fiber, and total amino acid contents, and in the amount of essential amino acids and the proportion of total unsaturated fatty acids and EPA. However, the Jindo population revealed the greatest carbohydrate, lipid, and mineral (Zn and Ca) contents of the three U. pinnatifida populations. In particular, Zn content of Jindo plants was 30 times greater than that of Kijang plants. Thus, the proximate composition, mineral composition, amino acid, and fatty acids of Undaria pinnatifida plants were distinguishable among the three representative Undaria populations evaluated. These results also indicate that these edible seaweeds contain significant nutritional value, such as protein and minerals, and that nutrient content varied according to environmental conditions of their habitat even within the same species (Dawes et al. 1993, Kaehler and Kennish 1996, Manivannan et al. 2008).
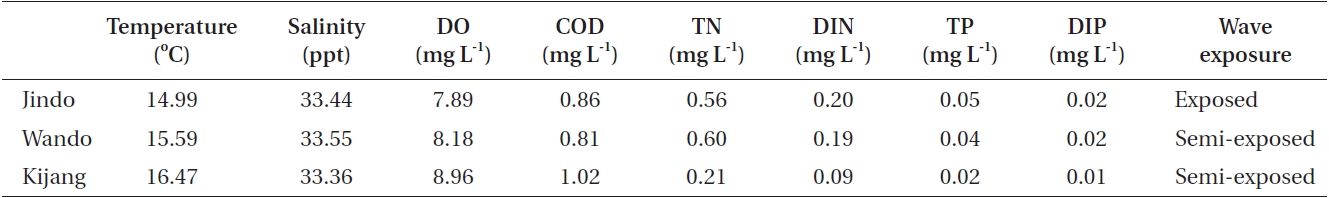
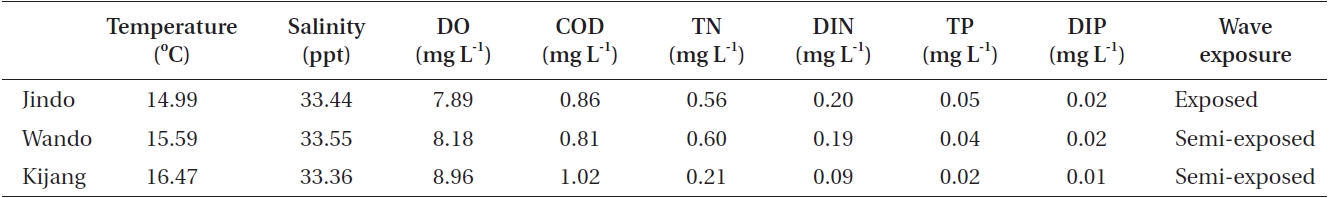
The morphological features and chemical composition of U. pinnatifida sporophytes varied among the three populations grown under different environmental conditions. Most environmental data were very similar among the study sites but total nitrogen, dissolved inorganic nitrogen, total phosphorous, and dissolved inorganic phosphorous were slightly higher at Jindo compared to those at Kijang (Table 6). The Jindo site was wave-exposed, whereas the Wando and Kijang sites were semi-exposed. In the present results, plant length, weight, and undivided blade width of U. pinnatifida sporophytes were smaller in the wave-exposed Jindo population than those in the semi-exposed Wando and Kijang populations. In previous studies, U. pinnatifida morphology changed due to environmental conditions (water depth, seawater temperature, and currents), and U. pinnatifida sporophytes are shorter and slimmer to survive in strong current areas (Saito 1960, Ii 1964). Thus, such different morphological and chemical composition are possibly attributed to differences in wave exposure, which changes plant morphology by direct physical conditions and changes the chemical composition of plants by indirectly providing different levels of inorganic nitrogen and phosphate .
However, frond length, the number of pinnae / frond length, and the number of sporophyll frills are stable morphological characters in U. pinnatifida transplant experiments, and do not change due to environmental factors (Saito 1972). Kito et al. (1981) reported that the stable morphological features related to the genetics of U. pinnatifida are midrib width and the length of the longest pinnate blade. Saito (1962) indicated that stipe length, total plant length, and the number of pinnate blades: total plant ratios are genetically fixed. In the present study, midrib width and plant length were significantly smaller at Jindo than those at Kijang, suggesting genetic differences between the two U. pinnatifida populations.
A genetic diversity study of Jindo and Kijang Undaria populations is required to understand whether their morphological differences originate from genetic differences. Additionally, proximate composition, mineral composition, amino acids, and fatty acids of U. pinnatifida were distinguishable among the three representative Undaria populations tested. Thus, we suggest that morphological and biochemical differences in the three U. pinnatifida populations might originate from different environmental conditions of their habitats.