Microalgae are photosynthetic organisms that convert carbon dioxide (CO2) into a wide spectrum of organic compounds including chlorophyll, carotenoid, vitamin and lipid. They are known as the primary producers of a variety of long-chain polyunsaturated fatty acids (PUFAs) in aquatic environments and numerous studies have shown that nutraceutically important omega-3 (ω3) and omega-6 (ω6) PUFAs are quite abundant in microalgae (Otle? and Pire 2001, Bigogno et al. 2002, Ikawa 2004, Patil et al. 2007, Khozin-Goldberg et al. 2011). As the global ω3 and ω6 PUFAs market is expanding at a remarkable rate, microalgae have gained considerable attention as an alternative source to fish oil due to their fast growth rate and high PUFA contents (Seto et al. 1984, Benemann et al. 1987). Therefore, the commercial cultivation of
>
Sample collection and isolation
Samples of garden soil were taken near the Biological Sciences Building at Kyungpook National University (35°53′ N, 128°36′ E) in Daegu, South Korea in May 2010. A few micrograms of fresh soil were used to inoculate 100 mL BG-11(+) medium containing nitrate (Rippka et al. 1979) with 100 μg mL-1 of meropenem (Yuhan Pharmaceuticals, Ochang, Korea). The flasks were incubated on an orbital shaker (Vision Scientific, Bucheon, Korea) at 160 rpm and 25℃ until algal growth was observed. Well-established algal cultures (1.5 mL) were centrifuged at 3,000 ×g for 15 min. The resulting pellets were streaked onto BG-11 agar supplemented with meropenem and incubated at 25℃ with a light : dark cycle (16 : 8 h). A single colony was then aseptically re-streaked onto a fresh BG-11 plate containing meropenem (20 μg mL-1) to obtain an axenic culture.
>
Morphological identification
The isolate was grown in BG-11(+) medium for 28 days. Live cells were harvested by centrifugation at 3,000 ×g for 5 min, washed with sterile distilled water, and inspected at 400× magnification with a Zeiss Axioskop 2 light microscope (Carl Zeiss, Standort Gottingen, Vertrieb, Germany) equipped with differential interference contrast (DIC) optics.
Genomic DNA was extracted by using a DNeasy plant mini kit (Qiagen, Hilden, Germany). The PCR conditions and primer sets NS1 (5′-GTA GTC ATA TGC TTG TCT C-3′) and NS8 (5′-TCC GCA GGT TCA CCT ACG GA-3′) used for 18S ribosomal RNA (rRNA) sequence analysis were previously described by White et al. (1990). Amplification
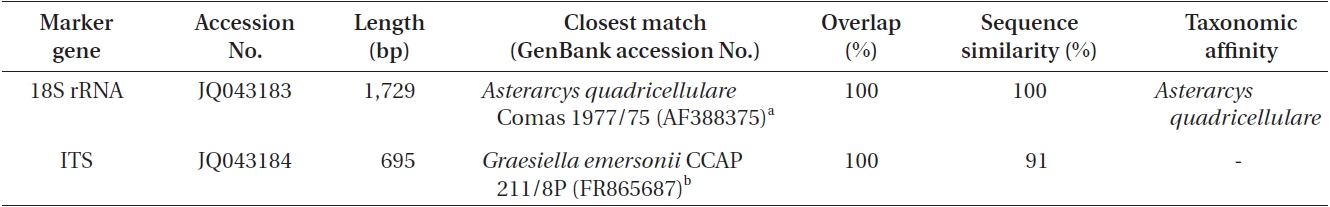
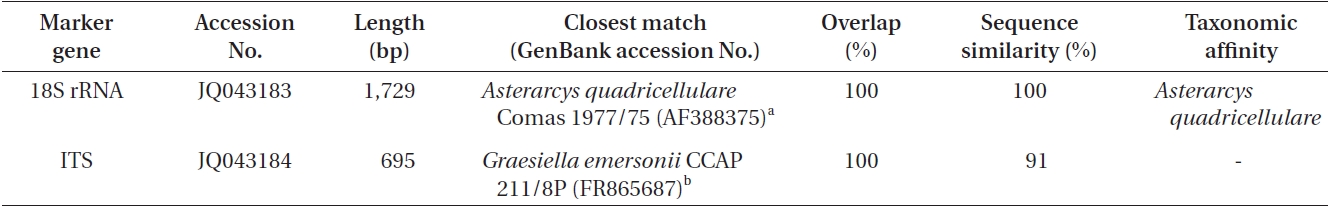
Results from BLAST searches using the 18S rRNA and ITS sequences of Asterarcys quadricellulare KNUA020
was performed in 25 μL reaction volume containing approximately 10 ng of template DNA, 0.5 μM of each primer (Macrogen, Seoul, Korea), 0.5 U of
The PCR products were purified using a LaboPass PCR purification kit (Cosmo Genetech, Seoul, Korea). The purified PCR amplicons were ligated into a pGEM Easy vector system (Promega, Madison, WI, USA) and used to transform
A 30-day-old seed culture of
>
Lipid extraction and gas chromatography/mass spectrometry (GC/MS) analysis
To simulate commercial production of microalgae-based biochemicals and obtain enough biomass for analysis, each seed culture was used to inoculate 16 L of a commercial liquid fertilizer (1 : 1,000 dilution; BioNex; 5.1% N, 10% P2O5, and 5% K2O; Biosangsa, Busan, Korea) in an 18-L transparent polycarbonate bottle in triplicate. The cultures were autotrophically grown at 25℃ with a flow of air bubbles at rate of approximately 2 L min-1 under cool fluorescent lighting (approximately 70 μmole m-2 s-1) with a light : dark cycle of 16 : 8 h. After incubating for 30 days, algal cells were harvested by centrifugation at 3,220 ×g (Centrifuge 5810R; Eppendorf, Hamburg, Germany) for 10 min. The harvested algal biomass was freeze-dried and mixed with chloroform-methanol (2 : 1) overnight according to the method described by Yeo et al. (2011). The chloroform extract was isolated and dried in a rotary evaporator (RV10; IKA, Wilmington, NC, USA). The crude lipid was weighed and treated with a pre-made solution of methanol and potassium hydroxide Hexane was added to the reaction and the entire mixture was heated to 30℃ and stirred for 10 h. The mixture was then cooled and the methanol and hexane layers were separated. The yellow hexane layer was isolated for further analysis. The fatty acid composition of the culture was determined by GC/MS (Jeol JMS700 mass spectrometer equipped with an Agilent 6890N GC; Agilent Technologies, Palo Alto, CA, USA) at the Daegu Center, Korea Basic Science Institute (KBSI). Peak identification and compound assignment were performed based on the electron impact mass spectrum (EI-MS). The National Institute of Standards and Technology (NIST) mass spectral libraries were used as reference databases.
>
Identification of the isolated microalga
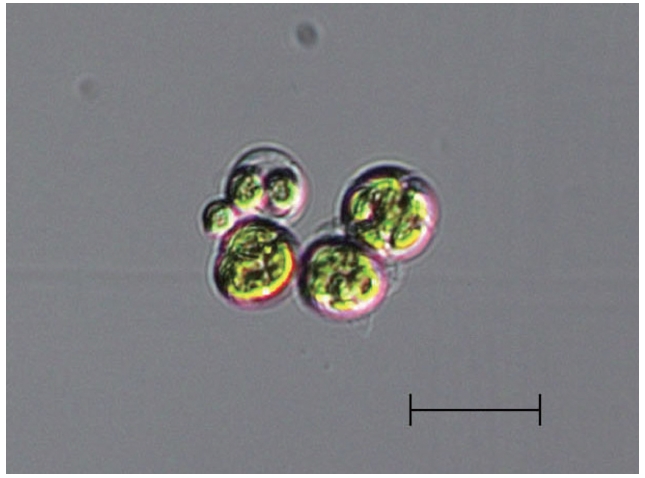
The algal isolate was non-motile with three-dimensionally arranged coenobia. Its diameter varied from 3-4 μm up to 20 μm depending on the growth stage (Fig. 1). The coenobia consisted of randomly distributed (1-, 2-,
[Fig. 1.] Light microscope image of Asterarcys quadricellulare KNUA020. Scale bar represents: 20 μm.
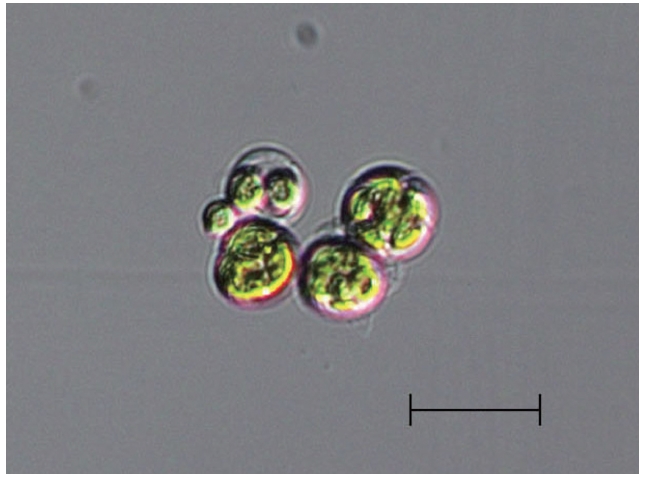
4-, or more) cells within a spherical mucilage envelope. However, it was very difficult to accurately classify the isolate by comparing morphological characteristics shared with other algae because only one species in this genus [
According to the 18S rRNA sequencing data (Table 1), the isolate had a 100% sequence homology with
>
Physiological properties of Asterarcys quadricellulare KNUA020
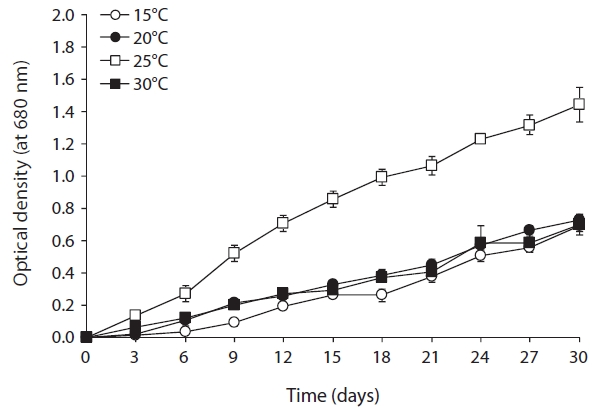
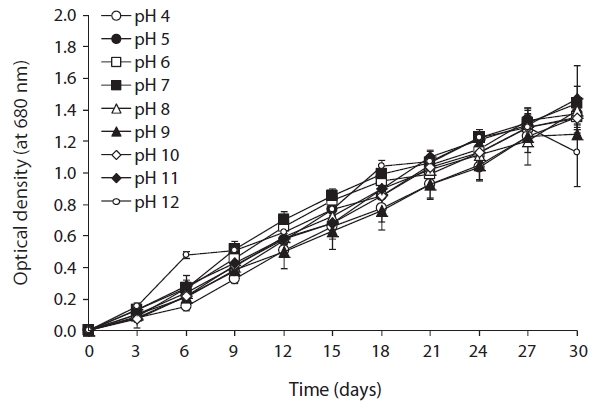
As shown in Fig. 2, optimal growth temperature for
strain KNUA020 was 25℃. These cells were able to grow and survive at 15, 20, and 30℃, but delayed growth was observed when they were incubated at these temperatures. The microalga also grew well across a wide pH range (pH 4.0-12.0) (Fig. 3) while maximal growth was obtained around pH 7.0 at 25℃. However, the autotrophic growth rate of strain KNUA020 was relatively low. To develop cost-effective algal biomass production, microalgae can be cultured in heterotrophic conditions where organic carbons such as sugars, sugar alcohols or organic acids serve as less expensive carbon sources (Pyle et al. 2008, Liang et al. 2009). As heterotrophic growth of strain KNUA020 in complex media such as LB and R2A was also observed in this study (data not shown) and a number of reports have demonstrated that heterotrophically grown microalgae accumulate high proportions of lipids (Miao and Wu 2006, Xiong et al. 2008, Gao et al. 2010), future work should be carried out to determine whether mixotrophic cultivation of strain KNUA020 results in both faster growth rate and increased lipid productivity.
>
Lipid extraction and GC/MS analysis
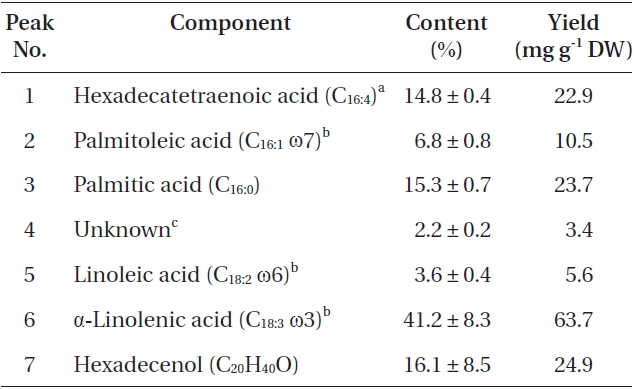
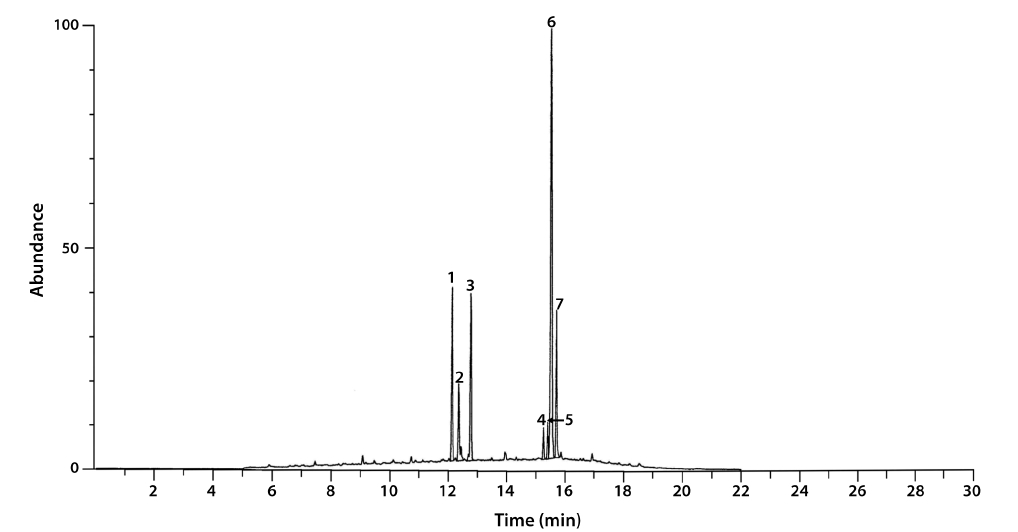
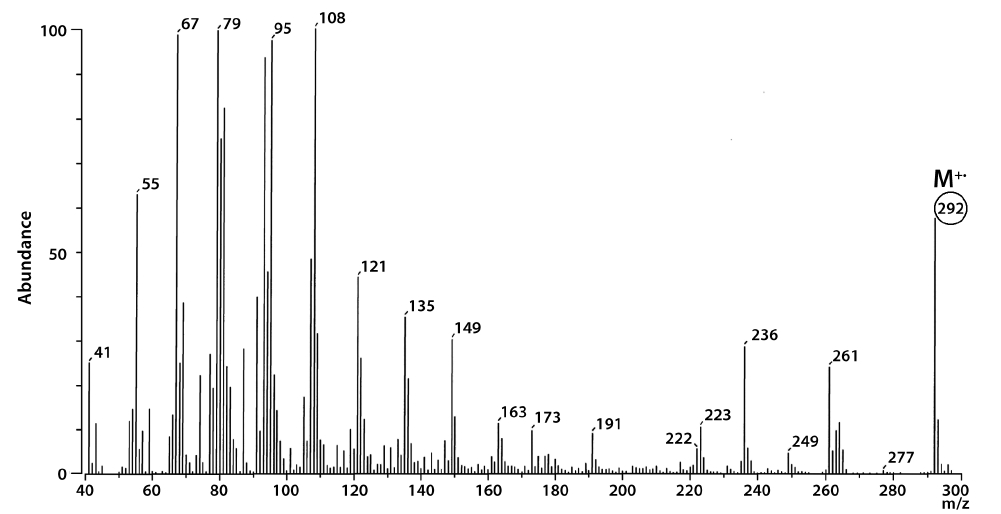
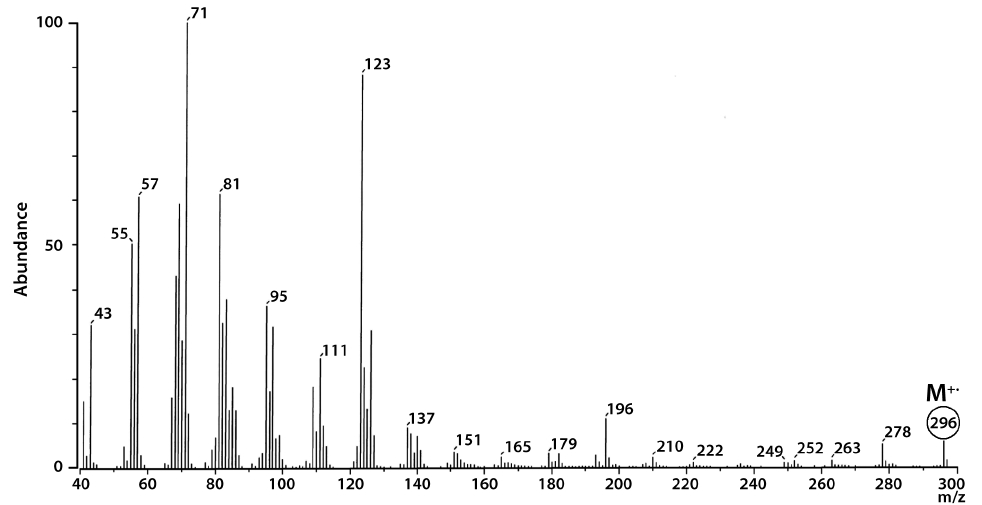
The total lipid content of the isolate was 15.5 ± 0.2% of dry weight (DW). The GC/MS results showed that the KNUA020 strain produced palmitic acid (C16:0, 15.3 ± 0.7%, 23.7 mg g-1 DW), hexadecatetraenoic acid (C16:4, 14.8 ± 0.4%, 22.9 mg g-1 DW) and α-linolenic acid (C18:3 ω3, 41.2 ± 8.3%, 63.7 mg g-1 DW) as major fatty acids (Table 2, Fig. 4). It was shown that the most abundant fatty acid produced by the KNUA020 strain was α-linolenic acid (C18:3 ω3, ALA, molecular weight = 292) which is a nutritionally important ω3 fatty acid (Table 2, Fig. 5). This isolate may thus have potential as an alternative ω3 PUFA source for fish oil. In addition, it was discovered that a significant amount of hexadecenol (C20H40O, molecular weight = 296, 24.9 mg g-1 DW) was also produced by this photosynthetic microorganism (Table 2, Fig. 6). Long-chain fatty alcohols have been widely used in the cosmetics and soap industries and have recently gained particular interest as biofuels to replace petroleum-derived compounds (Kalscheuer et al. 2006, Fortman et al. 2008, Rottig et al. 2010, Steen et al. 2010). Therefore, this algae-derived hexadecenol also has potential to be used as a petroleum additive.
>
Asterarcys quadricellulare KNUA020 as PUFA feedstock
As mentioned above, little is known about the microalga isolated in the present study since there is only one species in the genus
[Table 2.] List of fatty acids and fatty alcohol present in Asterarcys quadricellulare KNUA020
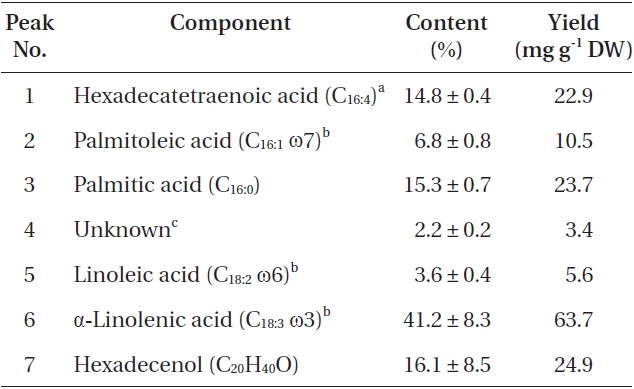
List of fatty acids and fatty alcohol present in Asterarcys quadricellulare KNUA020
logical characteristics and potential of
In the present study, a South Korean domestic microalga,