Seagrasses are known to play an important role as a primary producer in coastal and estuarine ecosystems (McRoy and McMillan 1977, Zieman and Wetzel 1980, Lee and Dunton 1996). Seagrasses can affect the coastal and estuarine environment by enhancing sedimentation and reducing current and wave energy (Fonseca 1989). Seagrasses also improve water qualities of coasts and estuaries by removing over-enriched inorganic nutrients through leaf uptake (Short and McRoy 1984, Lee and Dunton 1999). Moreover, seagrass meadows provide habitats for commercially and ecologically valuable marine organisms (Holmquist et al. 1989, Montague and Ley 1993). Since seagrass production is essential to support coastal and estuarine ecosystems, examination of seagrass growth dynamics is critical to understand functions and values of seagrass at a certain area in coastal and estuarine ecosystems.
The primary productivity of seagrass is usually controlled by underwater irradiance, water temperature, and inorganic nutrient availabilities (Wetzel and Penhale 1983, Dunton 1994, Lee et al. 2007
Since seagrasses achieve high level of production, they require high inorganic carbon and nutrient incorporation. Seagrasses can utilize both CO2 and HCO3 - for photosynthetic carbon reduction (Durako 1993, Larkum and James 1996, Beer and Rehnberg 1997, Invers et al. 2001). Because the CO2 supply in seawater can be restricted by low solubilities and diffusion rates, seagrasses have adapted to use HCO3 -, which is the most abundant dissolved inorganic carbon (DIC) in seawater (Larkum and James 1996, Beer and Rehnberg 1997, Bjork et al. 1997, Invers et al. 2001). However, the affinity for HCO3 - has been reported to be lower than that of CO2 (Bjork et al. 1997, Invers et al. 2001). Seagrasses store carbon products in rhizome tissue in the form of non-structural carbohydrate during high photosynthetic periods, and stored carbon in below-ground tissue then supports the growth and maintenance of plants during periods of low photosynthetic production (Dawes and Lawrence 1980, Durako and Moffler 1985, Pirc 1985, Dawes and Guiry 1992, Lee and Dunton 1996). Therefore, production, metabolism and stored carbon content of below-ground tissues may be important factors for estimation of whole plant carbon balance (Fourqurean and Zieman 1991, Kraemer and Alberte 1993).
Seagrasses also require large amounts of inorganic nutrients, especially nitrogen, to sustain their high productivities (Kenworthy et al. 1982, Hemminga et al. 1991, Blackburn et al. 1994). The major nitrogen sources available to seagrasses include NH4 + and NO3 - + NO2 - in the water column and sediment pore water. Seagrasses can take up inorganic nitrogen through both leaf and root tissues, often with equal uptake contributions from water column and sediment nutrients (Short and McRoy 1984, Pedersen et al. 1997, Terrados and Williams 1997, Lee and Dunton 1999). Because dissolved inorganic nitrogen (DIN) concentrations are usually higher in sediment pore water than in the water column, sediment DIN is often considered to be the main nitrogen source for seagrass growth (Short and McRoy 1984, Zimmerman et al. 1987). However, some studies have suggested that seagrass leaves have higher nutrient affinities compared with roots to assimilate nutrients under lower concentrations (Pedersen et al. 1997, Lee and Dunton 1999). Thus, although there are significant differences in nutrient concentrations between the water column and sediment pore water, they equally contribute to seagrass nutrient acquisition for seagrass growth (Stapel et al. 1996, Terrados and Williams 1997, Lee and Dunton 1999). Although seagrasses can utilize nitrogen from both the water column and sediments, reduced seagrass tissue N content has been reported during the periods of high growth (Stocker 1980, Pirc and Wollenweber 1988, Perez-Llorens and Niell 1993). Thus, we hypothesized that seagrass growth is probably limited by inorganic C and N during high growing periods at the study sites.
Eelgrass,
The two study sites (Myungju and Dagu stations) were located in Jindong Bay on the southern coast of Korea (Fig. 1). This study was conducted on a monotypic meadow of
>
Measurements of environmental factors
Water temperature and underwater PFD were measured every 15 min during the experimental period using a Hobo data logger (Onset Computer Corp., Bourne, MA, USA) encased in a waterproof underwater housing. Measured water temperatures were averaged daily. Light intensity (lumens ft-2) measured using a Hobo data logger was converted to photon flux density (μmol photons m-2 s-1) by concurrent quantum measurements using an LI-1400 data logger and an LI-193SA spherical quantum sensor (LI-COR Inc., Lincoln, NE, USA). Daily photon flux density (mol photons m-2 d-1) was calculated as the summation of quantum flux over each 24 h period. Four replicate surface water samples were collected to determine water column DIN (NH4 + and NO3 - + NO2 -) concentrations. DIN concentrations were determined colorimetrically according to the methods of Parsons et al. (1984). Concentrations of NO3 - + NO2 - were determined after passing the samples through a column containing copper coated cadmium, which reduces NO3 - to NO2 -. Six to ten replicate sediment samples were collected randomly, to a sediment depth of about 13 cm using a corer for measurement of sediment pore water DIN concentrations. Samples were placed on ice and frozen pending lab analysis. Sediment pore water was obtained by centrifugation and pore water DIN concentrations were measured after dilution with low nutrient seawater.
>
Leaf productivity and tissue C and N content
Leaf productivity was determined by the leaf marking technique (Zieman 1974, Kentula and McIntire 1986).
Ten randomly chosen shoots from each site were marked just above the bundle sheath with a hypodermic needle and collected after a period of 2-4 weeks. Leaf materials were separated into leaf tissues produced before and after marking. Leaf productivities were determined by dividing the dry weight of new leaf tissue produced after marking by the number of days since marking. The second or third youngest leaves as well as rhizome tissues from the first to sixth nodes were used to measure eelgrass tissue C and N content. The dried tissues were ground using mortars and pestles. Approximately 1-2 mg of ground tissue was put into tin boat to determine eelgrass tissue C and N content using a CHN elemental analyzer (Flash EA 1112; Thermo Finnigan, Milan, Italy) and C : N molar ratios were then calculated.
All values were reported as means ± 1 standard error (SE). Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Data were tested for normality and homogeneity of variance to meet the assumptions of parametric statistics. Significant differences in eelgrass tissue C and N content, and leaf productivities between sampling stations and among sampling time were tested using a two-way ANOVA. When a significant difference among variables was observed, the means were analyzed by a Tukey multiple comparison test to determine where the significant differences occurred. Correlation analyses were used to examine the relationships between leaf productivity and tissue C and N content.
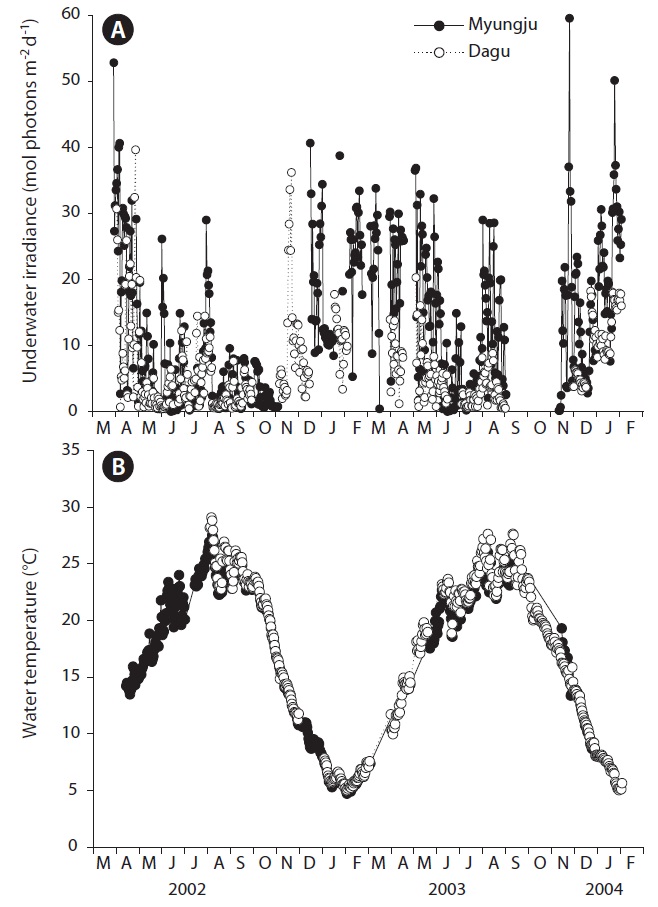
Daily underwater PFD significantly (p < 0.001) changed with sampling time, but did not show a distinct seasonal trend (Fig. 2A). Daily underwater PFD was usually lowest in summer at both study sites. Underwater PFD in Myungju station was significantly (p < 0.001) higher than that in Dagu station. Water temperature at both study sites significantly (p < 0.001) changed with sampling time, and showed a distinct seasonal trend (Fig. 2B). There was no significant (p = 0.163) difference in water temperature between Myungju and Dagu stations.
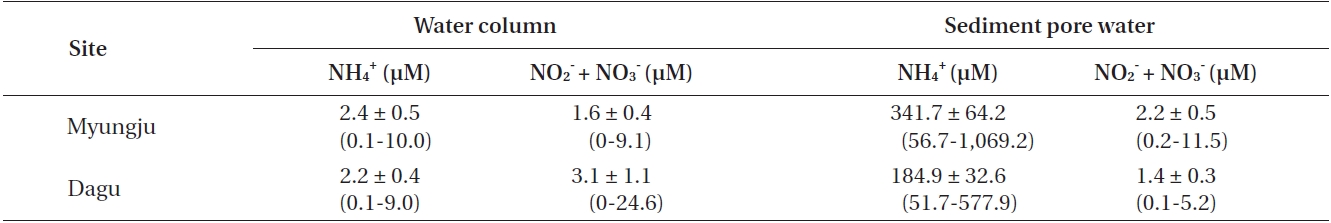
Water column NH4 + concentrations were not significantly (p = 0.306) different between the two study sites and were usually less than 3 μM at both sites (Table 1). Water column NO3 - + NO2 - concentrations were significantly (p < 0.001) different between the two study sites, and were usually less than 5 μM at both sites (Table 1). Sediment pore water NH4 + concentration at Myungju station was significantly (p < 0.001) higher than that at Dagu station (Table 1). Sediment pore water NH4 + concentrations were usually less than 300 μM. Sediment pore water NO3 - + NO2 - concentrations were also significantly (p < 0.001) higher at Myungju than at Dagu station, and were usually less than 2 μM at both sites (Table 1).
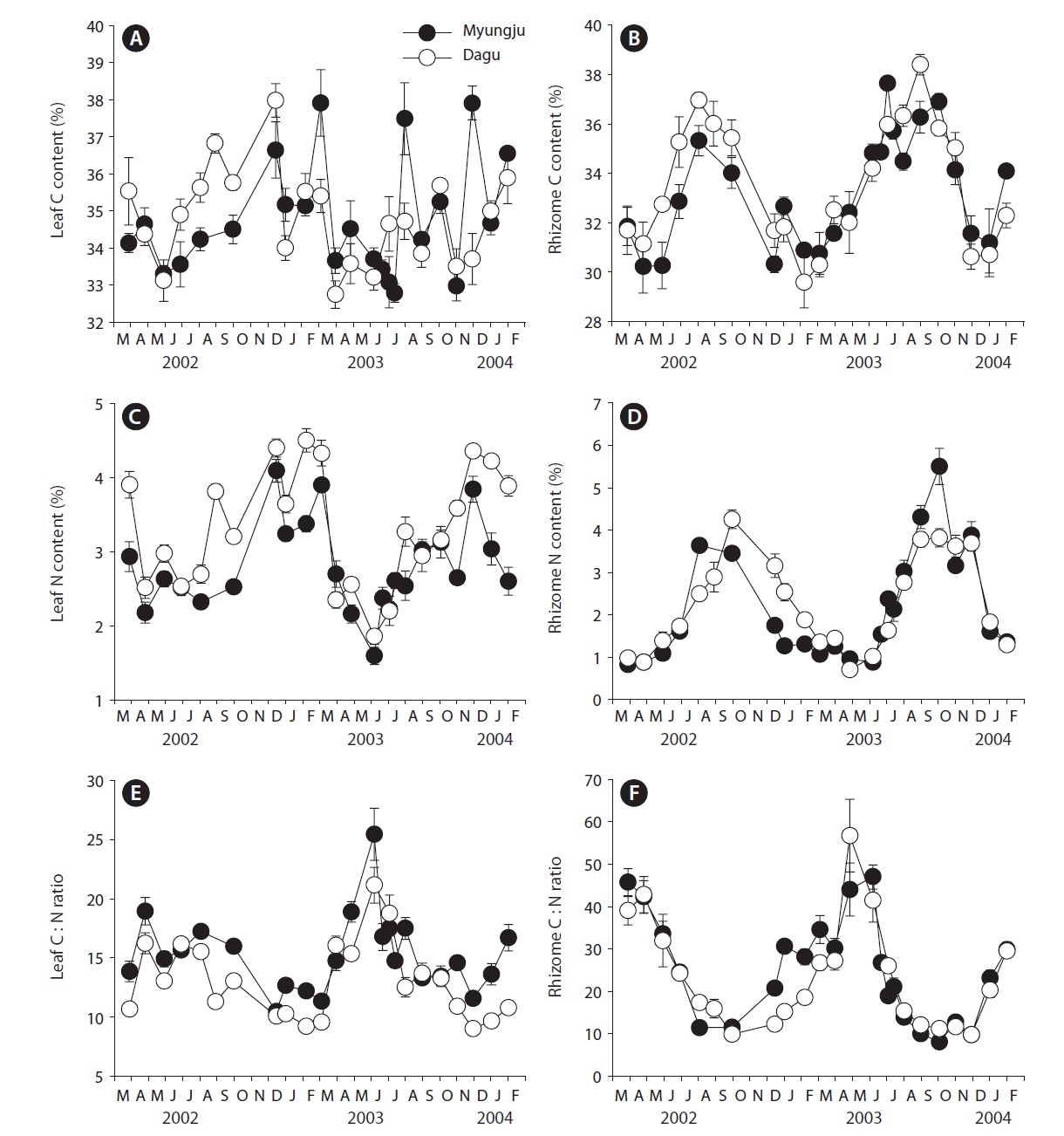
Leaf C content at both study sites showed significant (p < 0.001) seasonal variation, increasing during fall and winter and decreasing in spring, but did not show significant (p = 0.163) difference between the two study sites (Fig. 3A). Rhizome C content also exhibited significant (p < 0.001) seasonal variation, being highest during summer and early fall and lowest during winter at both study sites (Fig. 3B). Rhizome C content at Dagu station was significantly (p < 0.001) higher than that at Myungju sta-
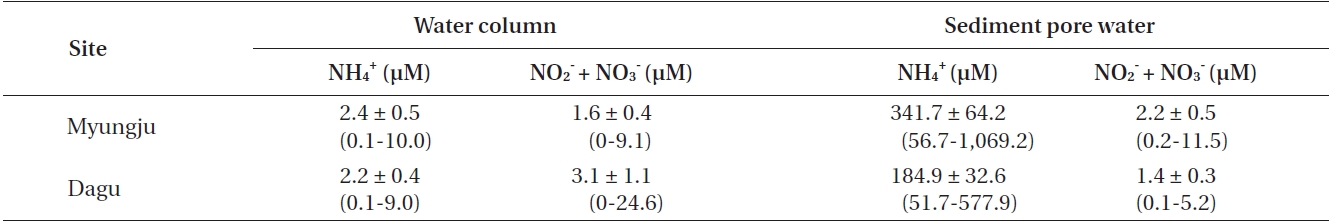
Average concentrations and ranges of dissolved inorganic nitrogen in the water column and sediment pore water at the two study sites in Jindong Bay from March 2002 to January 2004
tion. Leaf N content at both study sites showed significant (p < 0.001) seasonal trends, being highest in winter and lowest in summer, and the N content at Dagu station was significantly (p < 0.001) higher than that at Myungju station (Fig. 3C). Rhizome N content also showed significant (p < 0.001) seasonal trend, being highest in fall and lowest in spring (Fig. 3D). Rhizome N content at Dagu station was slightly higher than that at Myungju station. Eelgrass leaf C : N ratios showed significant (p < 0.001) seasonal-
ity, being highest during spring and summer and lowest during fall and winter at both study sites, and the ratio at Myungju station was significantly (p < 0.001) higher than that at Dagu station (Fig. 3E). Rhizome C : N ratios also showed significant (p < 0.001) seasonal trends, being highest in spring and lowest in fall at both study sites, and the ratio at Myungju station was slightly higher than that at Dagu station (Fig. 3F). Leaf and rhizome C : N ratios exhibited reverse trends of leaf and rhizome N content, respectively.
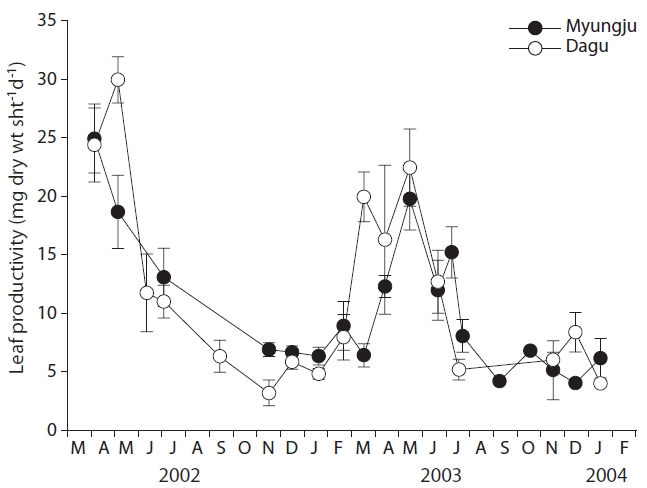
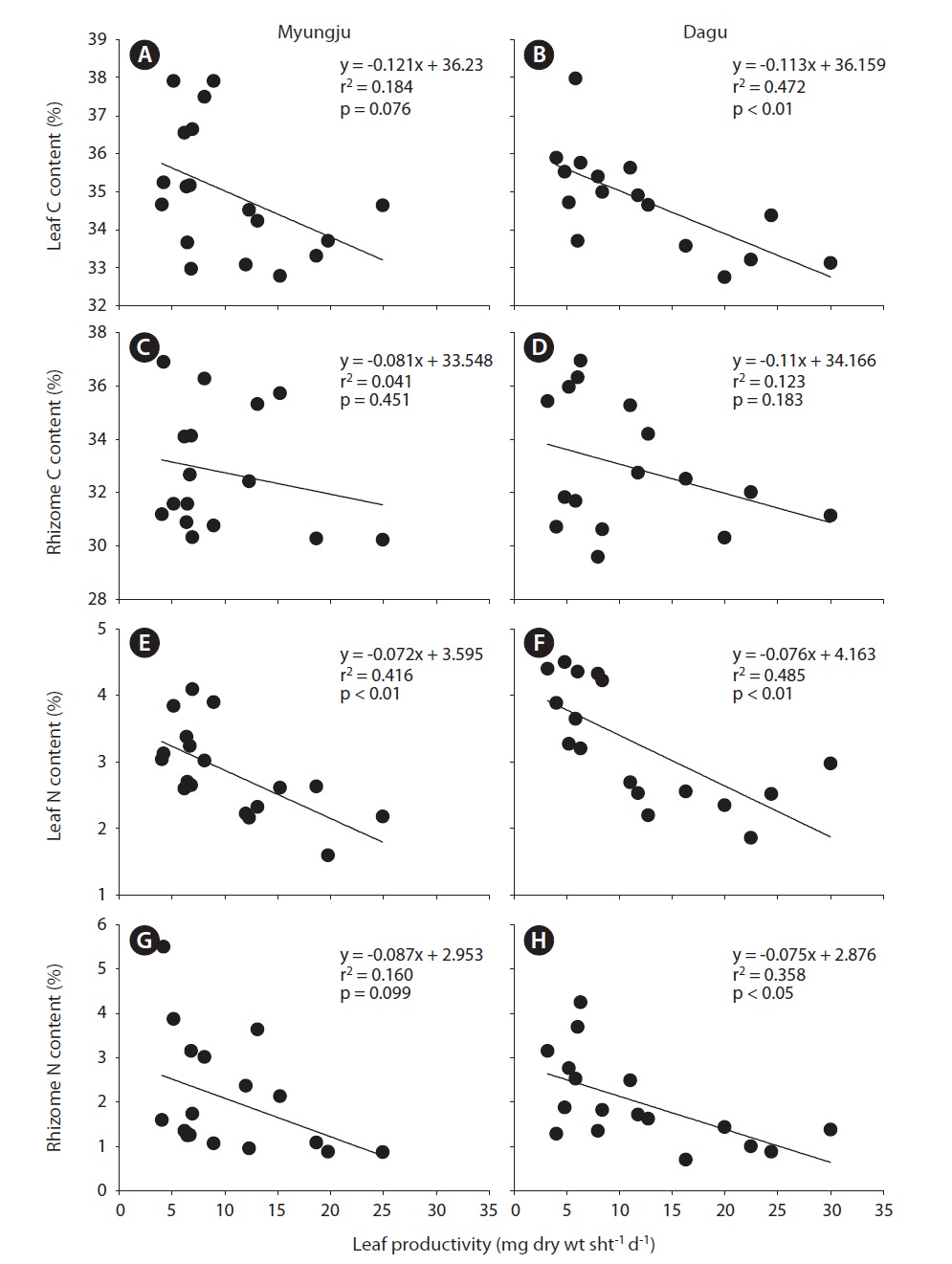
Eelgrass leaf productivities at both study sites showed a significant (p < 0.001) seasonal trend, increasing during spring and decreasing during fall and winter (Fig. 4). Leaf productivity exhibited similar seasonal variation at the two study sites. Leaf productivities were negatively correlated with tissue C and N content at both study sites (Fig. 5). Both leaf and rhizome tissue C and N content were usually lower during periods of high leaf productivity at
the two study sites. Slopes and y-intercepts of the regression lines between leaf productivity and tissue C and N content were similar between the two study sites (Fig. 5).
>
Factors controlling Zostera marina growth
The primary productivity of seagrass is usually controlled by underwater irradiance, water temperature, and inorganic nutrient availabilities (Wetzel and Penhale 1983, Dunton 1994, Lee et al. 2007
Seagrass growth may be highest at optimal temperature, determined by the balance between respiration and photosynthetic rates (Marsh et al. 1986). Lee et al. (2005) reported that the optimal temperature for
>
Zostera marina growth and tissue C content
Seagrass, as a primary producer, requires large amounts of inorganic carbon to sustain growth and survival (Duarte 1990, Fourqurean et al. 1997, Lee and Dunton 1999). In contrast to terrestrial plants, seagrasses obtain their carbon from seawater, which has high inorganic carbon concentrations (Sand-Jensen 1977). However, the CO2 supply for photosynthesis in seawater may be restricted by low solubility and diffusion rates (Bjork et al. 1997), and thus most of seagrasses utilize HCO3 -, which is the most abundant form of DIC in seawater (Durako 1993, Larkum and James 1996, Beer and Rehnberg 1997, Invers et al. 2001). HCO3 - acquisition into seagrass photosynthetic tissues usually involves extracellular carbonic anhydrase for the formation of CO2 from HCO3 - prior to uptake (James and Larkum 1996, Beer and Rehnberg 1997, Bjork et al. 1997, Invers et al. 2001). Thus, the affinity of HCO3 - is lower than that of CO2 for seagrass photosynthesis. Since the most abundant DIC has low affinity, inorganic carbon limitation for seagrass photosynthesis is probably a common feature during high growing seasons, and / or in stagnant water conditions. In the present study, leaf and rhizome C content exhibited negative correlations with leaf productivity at the two study sites (Fig. 5A-D). These negative correlations between leaf productivity and tissue C content imply that there was a carbon limitation for seagrass photosynthesis in these study sites.
Carbon products produced by photosynthesis are reserved in seagrass rhizome tissue in the form of soluble carbohydrate carbon, which is used to support growth and maintenance of plants during periods of low photosynthetic production (Dawes and Lawrence 1980, Durako and Moffler 1985, Dawes and Guiry 1992). In the present study, leaf C content at both study sites showed seasonal trends, increasing during fall and winter and decreasing during spring (Fig. 3A). Rhizome C content was also lowest in early spring when new
bon in seagrass tissues was probably utilized to support growth and new lateral shoot formation during periods of low photosynthetic production.
>
Zostera marina growth and tissue N content
Since seagrasses have high productivities, they require a large amount of nutrients, especially inorganic nitrogen (Kenworthy et al. 1982, Hemminga et al. 1991, Blackburn et al. 1994). Thus, nutrient availability in seagrass habitats plays an important role in controlling seagrass growth. Seagrasses take up NH4 + and NO3 - + NO2 - from the water column through leaves and these from sediment pore water through rhizome and roots (Short and McRoy 1984, Pedersen et al. 1997, Terrados and Williams 1997, Lee and Dunton 1999). NH4 + uptake rates are usually higher than NO3 - + NO2 - in seagrasses (Terrados and Williams 1997, Lee and Dunton 1999). Therefore, seagrasses usually utilize NH4 + from both the water column and sediments as a major nitrogen source. In this study, eelgrass leaf N content at both study sites showed significant seasonal trends, increasing during summer and fall and decreasing during winter and spring. Leaf N content and leaf productivities exhibited significant (r2 = 0.416, p < 0.01 in Myungju station; r2 = 0.485, p < 0.01 in Dagu station) negative correlations at both study sites. Eelgrass rhizome N content at both study sites also showed significant seasonal variations, increasing during summer and decreasing during winter and spring. Rhizome N content and productivities showed negative correlations at both study sites, and the relationship was significant (r2 = 0.358, p < 0.05) at Dagu station. These negative correlations between tissue N content and productivity suggest that
Many studies have reported negative correlations between seagrass growth and tissue N content (Stocker 1980, Pellikaan and Nienhuis 1988, Pirc and Wollenweber 1988, Perez-Llorens and Niell 1993, Short et al. 1993). Short et al. (1993) demonstrated that high growth rates can cause N depletion from the root zone in sediments, which may result in nutrient limitation and consequently lower nutrient content in seagrass tissues. Seasonal variation in tissue N content has also been explained by seasonal differences in N uptake rates and dilution processes (Stocker 1980, Pellikaan and Nienhuis 1988, Pirc and Wollenweber 1988, Perez-Llorens and Niell 1993). N uptake may exceed seagrass N requirements during periods of low growth, and the excess N is then stored in tissues leading to high tissue N content (Thursby and Harlin 1982). During periods of high growth, on the other hand, seagrass tissue biomass increases, and tissue N may reallocate to the new tissue. Thus, low tissue N content during periods of high growth may be a product of N dilution according to rapid seagrass growth.
In summary, productivities of eelgrass,