Aquatic macrophytes, often also called hydrophytes, are key components of aquatic and wetland ecosystems. As primary producers, they are at the base of herbivorous and detritivorous food chains, providing food to inver-tebrates, fish and birds, and organic carbon for bacteria. Their stems, roots and leaves serve as a substrate for pe-riphyton, and a shelter for numerous invertebrates and different stages of fish, amphibians and reptiles (Timms and Moss 1984, Dvo?ak 1996). Biogeochemical processes in the water column and sediments are to a large extent influenced by the presence/absence and a type of mac-rophytes, and macrophytes can also have a profound im-pact on water movement and sediment dynamics in wa-ter bodies. Some macrophytes are of major importance for their direct contributions to human societies by pro-viding food, biomass, and building materials (Costanza et al. 1997, Engelhardt and Ritchie 2001, Egertson et al. 2004, Bornette and Puijalon 2011).
Good knowledge of the functions of aquatic macro-phytes in wetlands and shallow lake ecosystems is critical for understanding the basic ecosystem processes. It is also important for numerous applied issues such as wetland restoration, wastewater treatment, and management of invasive species (Lavoie 2010, Casanova 2011).
The goal of this review is to briefly summarize various macrophyte classifications, and cover in more details nu-merous aspects of the macrophytes’ role in aquatic and wetland ecosystems.
The diverse and heterogeneous group of macrophytes has posed a challenge for definition and classification. Macrophytes can be loosely defined as all forms of mac-roscopic aquatic vegetation visible by naked eye. This is in contrast to microphytes, i.e., microscopic forms of aquatic plants, such as planktonic and periphytic algae. The fact that macrophytes live in aquatic environments, at least seasonally, makes them different from terrestrial plants that don’t tolerate flooded environments. The macrophytes include taxonomically very diverse repre-sentatives: macroalgae (e.g.,
There are a number of classical treaties on aquatic macrophytes (Gessner 1955, Sculthorpe 1967, Hutchin-son 1975, Cook 1996) some of them presenting quite elab-orated classification schemes (Hejny 1960, Den Hartog and Segal 1964). Hutchinson (1975) attempted to consoli-date various life-form based classifications (grouped by the relation of plants to water level and substratum) and growth-form based classifications (grouped by structural similarity and relations to the physical environment) into a unified “ecological” classification. Although his product is quite detailed, its main four categories remain the most widely accepted macrophyte classification until today (Wetzel 1975, Denny 1985). It differentiates between freely floating macrophytes and those attached to the substrate, with the attached, or rooted macrophytes further divided into three categories: floating-leaved, submerged and emergent. Brief description of the 4 categories follows:
“Emergent macrophytes” typically occur in the upper littoral zone at a depth of about 1-1.5 m, their root and rh-zome systems are often adapted to permanently anaero-bic sediments and they have aerial reproductive organs. This group includes rather diverse types of plants that can be further categorized into two groups: 1) erect emergents (e.g.,
“Floating-leaved macrophytes” represented by genera such as
“Submersed macrophytes” (
“Freely floating macrophytes” (
Besides the Hutchinson’s ecological classification of macrophytes, several authors attempted to classify mac-rophytes into functional types (Boutin and Keddy 1993, Brock and Casanova 1997, Weiher et al. 1998). Plant func-tional types can be defined as sets of plants exhibiting similar responses to environmental conditions and hav-ing similar effects on the dominant ecosystem processes (Diaz and Cabido 1997, Lavorel et al. 1997). Grouping plants based on their functional traits became increas-ingly important when the need for understanding the link between plant species richness and ecosystem func-tioning has emerged as one of the central questions in ecology during the past decade (Bouchard et al. 2007). A simplification from species to functional groups is also attractive for predictive modeling of vegetation respons-es to human-induced changes in the environment, e.g., climate change (Nygaard and Ejrnaes 2004). However, despite different classification schemes and terminolo-gies there is no single commonly accepted functional type classification useful for all studies whether they are concerned with wetland or terrestrial plants (Sieben et al. 2010). It seems that plant functional groups are being de novo defined for each individual study depending on the study aims (Rejmankova et al. 2011).
Previous assessments of macrophyte diversity between temperate and tropical regions indicated that the rich-
ness was similar, or even richer, in temperate regions (Crow 1993).
However, Chambers et al. (2008) in their thorough comparison of global diversity of freshwater aquatic mac-rophytes among bioregions concluded that vascular mac-rophyte generic diversity for the tropics is greater than for temperate regions. These authors also pointed out that large gaps still exist in our knowledge of aquatic mac-rophyte abundance and distribution, and warned that many of the threats to fresh waters such as eutrophica-tion and alien species introductions will lead to reduced macrophyte diversity.
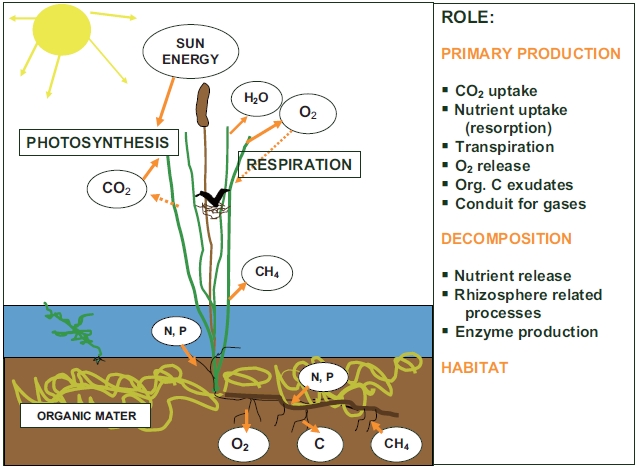
Different roles of macrophytes in wetland ecosystem, briefly mentioned in the introductory paragraph, are summarized in Fig. 1. The following chapter will explain the individual roles stressing the role of macrophytes in nutrient cycling.
Primary production is one of the basic ecosystem prop-erties. It represents the biomass (usually denoted as W) produced by plants per unit of area per unit of time. Of the most interest is the net primary production, NPP, defined as NPP = gross primary production - respiratory losses. NPP is expressed in units of dry mass per area per unit of
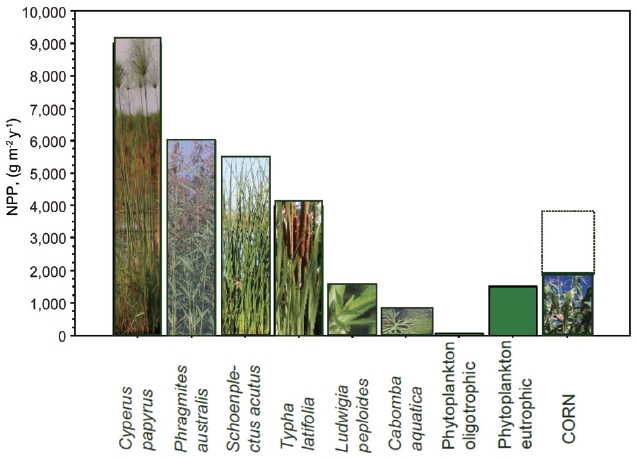
time, e.g., g dry mass m-2 y-1, sometimes also as ash free dry weight, or weight of carbon, C. NPP corresponds well to the maximum seasonal biomass (Wmax) for plants with one generation time, typically in temperate zones; other-wise it depends on generation time, in the tropics usually NPP ~2 to 3 times Wmax. Examples of NPP of various types of macrophytes are in Fig. 2.
As in terrestrial ecosystems, NPP depends on tempera-ture, solar radiation, and available nutrients. When tem-perature and solar radiation are near optimal, the ecosys-tem primary production is often limited by shortage of water and/or nutrients (Lambers et al. 1998). Water short-age in permanently flooded wetlands is not an issue and this explains why nutrient rich wetlands are among the most productive ecosystems in the world (e.g., papyrus swamp, see Fig. 2). Another example of highly productive macrophytes are grasses comprising so called floating meadows that develop on floodplains of tropical rivers during the rainy season such as the Amazon floodplain in Brazil, or Magela Creek floodplains in Australia where the peak above-ground biomass often exceeds 4,000 g/m2 (Junk 1997, Pettit et al. 2011).
Water depth and degree of anaerobiosis of flooded sediments may exert different impact on different species often depending on whether the air is moving through plants from the aerial organs to roots and rhizomes by simple diffusion (most species) or by pressurized ventila-tion (see further in the subchapter on gas exchange).
NPP can be also impacted by salinity and different types of macrophytes evolved different mechanisms for dealing with increased salinity level, i.e., for keeping their internal osmotic potential in a favorable range (Adam 1990). Some macrophytes decrease their internal osmotic potential by higher mineral salt uptake (
Although most wetlands are faced with a slower or faster eutrophication, there are still many places where the wetlands are experiencing nutrient limitation, mostly by nitrogen, N, or phosphorus, P, or their combination (Verhoeven et al. 1996, Gusewell and Koerselman 2002). The range of ombrotrophic to minerotrophic nutrient settings reflects a general trend from low to high nutrient availability, and increasing productivity along the ombro-trophic to minerotrophic gradient (Bedford et al. 1999, Venterink et al. 2003); for definition of wetland types see Mitsch et al. (2009). Thus prevailing N limitation is typical for vascular species of swamp and marsh communities (Bedford et al. 1999, Willby et al. 2001), with P limitation and co-limitation associated with more infertile bog and fen communities (Willby et al. 2001), or marshes with P-poor soils in carstic or serpentine regions (Rejmankova 2005, Sorrell et al. 2011). This gradient in P to N limitation is also associated with changes in species composition from stress-tolerators maximizing nutrient conservation, to fast-growing species that maximize growth and form tall, monospecific stands (Sorrell et al. 2011).
Shifts from N limitation to P can occur as a result of high atmospheric N deposition (Bobbink et al. 2010). These shifts may affect species composition and diver-sity (Bedford et al. 1999, Gusewell 2004) and impact many ecosystem processes.
>
Strategies for coping with nutrient limited environment
Plants have evolved two broad strategies for P acquisi-tion in nutrient-limiting environment: 1) “conservation of use;” and 2) “enhanced acquisition or uptake” (Vance et al. 2003, Ticconi and Abel 2004, Richardson et al. 2009).
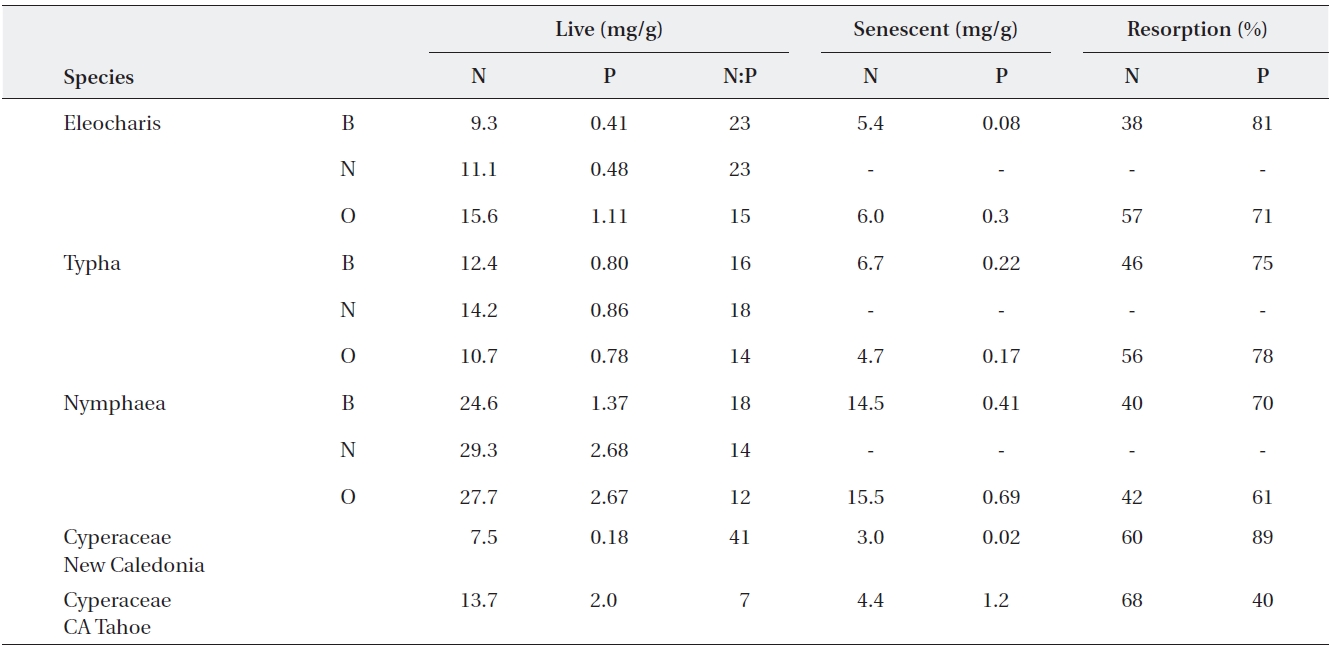
Resorption of nutrients from senescing to newly grow-ing or storage organs is a typical example of a conserva-tion strategy (Aerts and Chapin 2000). Growth of pe-rennial plants is determined not only by the amount of nutrients they acquire, but also by the amounts of stored nutrients that can be reused. The relationship between nutrient content (N and P) in live tissues and in litter is dependent on resorption, i.e., removal of nutrients from senescing plant tissues and transport of these nutrients to storage organs or growing tissues (Lambers et al. 1998). Two characteristics commonly used to describe nutrient resorption are: 1) resorption efficiency, RE, which is the percent of nutrient reduction between green and senes-cent leaves, and 2) the terminal content of a nutrient in senescent leaves, termed also resorption proficiency (Kill-ingbeck 1996). While average phosphorus RE is around 55% (Aerts 1996), it can reach over 80% in P limited en-vironments (Feller et al. 1999, 2002, Gusewell 2005, Re-jmankova 2005) (Table 1). The average values for nitrogen resorption are slightly lower, 50%, and nitrogen RE never reaches as high values as phosphorus RE. Generally, N in plant tissue fluctuates less as compared to P (Foulds 1993, Aerts and Chapin 2000). Values of 3 mg/g and 0.1 mg/g are given as the ultimate terminal content of N and P, respec-tively, in senescent leaves (Aerts and Chapin 2000). The evidence suggests that the terminal content of nutrients in fully senesced leaves is a particularly sensitive indicator of nutrient availability in wetlands (Rejmankova 2005).
N/P ratio in live tissue is a good predictor of both P resorption efficiency and P resorption proficiency (Re-jmankova 2005). Phosphorus deficiency can lead to an incomplete N resorption (Feller et al. 2002) and about 6% increase in nitrogen RE of
The degree to which nutrients are resorbed from se-nescing material determines the quality of litter, which further impacts decomposition rates and the overall sedi-ment nutrient cycling (Aerts and Chapin 2000, Cai and Bongers 2007). Given this, long term changes in plant composition need to be considered when evaluating plant-mediated effects on ecosystem nutrient cycling fol-lowing eutrophication.
The enhanced acquisition of N, or P is characterized by a production and secretion of hydrolytical enzymes in-volved in hydrolysis of the respective organic compounds. Enzymes called phosphatases are responsible for release of P from organic P-esters (Duff et al. 1994, Vance et al. 2003, Ticconi and Abel 2004). Extracellular phosphatases are usually located on the outer surface of epidermal cells and root apical meristems (Vance et al. 2003) and the in-duction of phosphatases during P-deficiency is a univer-sal response in higher plants (Raghothama 1999, Phoenix et al. 2004). Typical representatives of enzymes hydrolyz-ing organic N compounds are peptidases (Nausch and Nausch 2000). As is the case with the phosphatases, sev-eral studies demonstrate increasing the peptidase activity with declining N availability (Nausch and Nausch 2000, Hill et al. 2006).
Another example of enhanced nutrient acquisition involves an increase in spatial soil exploration by expan-sion of the root systems, and formation of symbiotic asso-ciations of roots with mycorrhizae (Lambers et al. 1998). Under nutrient-limited conditions, arbuscular mycorrhi-zae, AM, typically enhance plant growth by providing the plant host with P in exchange for carbon resources (Hart et al. 2003, Holdredge et al. 2010). Until recently, arbus-cular mycorrhizal fungi were considered unimportant in wetlands because anoxic conditions associated with wa-terlogged soils were considered limiting for fungi that are obligate aerobes and have diminished activity in reduced environments (Peat and Fitter 1993). However, more and more studies bring the evidence that AM fungi are pres-ent and widespread in many wetlands, and may influence wetland plant community structure (Brown and Bledsoe 1996, Hildebrandt et al. 2001, Stevens and Peterson 2007, Lagrange et al. 2011). Several wetland plant species (Cy-peraceae, Chenopodiaceae, and Plumbaginaceae) that were thought to be non-mycorrhizal have been shown to have high levels of AMF colonization (Hildebrandt et al. 2001, Holdredge et al. 2010, Kandalepas et al. 2010). It is
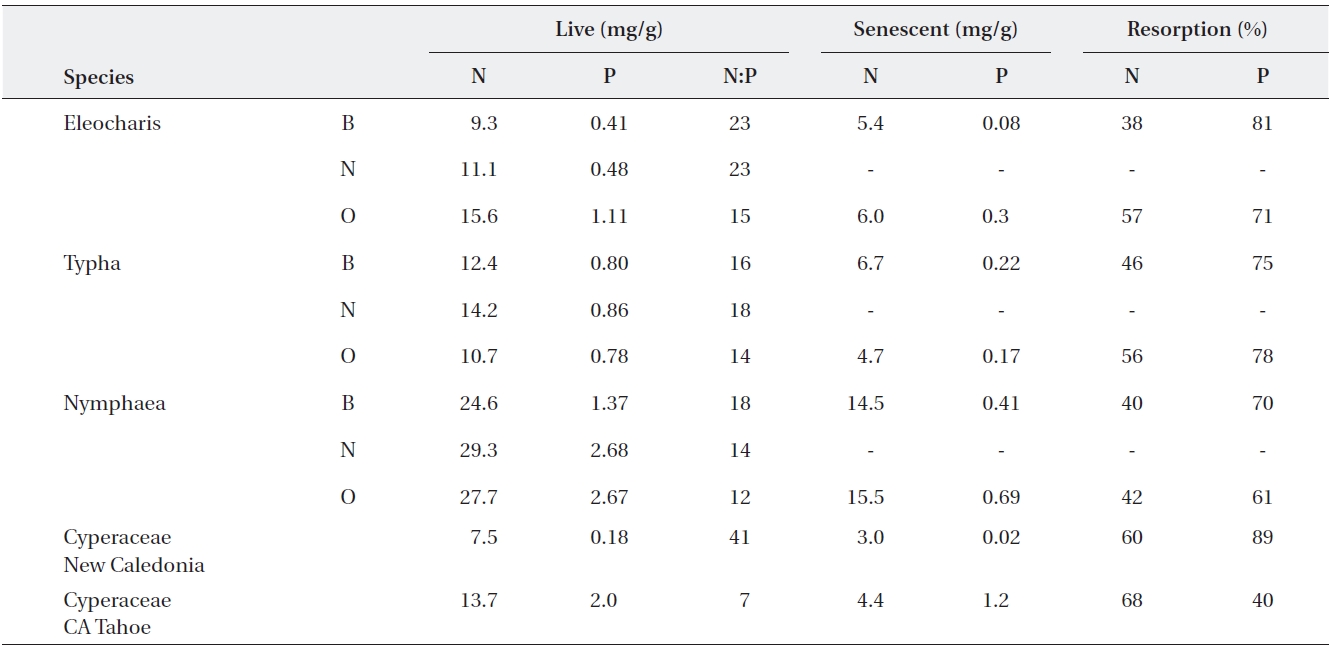
Examples of live and senescent tissue nutrients, and resorption efficiency in macrophytes from several regions
because wetland plants have the ability to oxygenate the rhizosphere (Armstrong et al. 1994) and this may mitigate the negative impact of flooding on the rhizosphere asso-ciated AM fungi (Ipsilantis and Sylvia 2007). Generally, the fungal colonization is higher in dicots than in monocots, with Cyperaceae generally showing a low colonization (Weishampel and Bedford 2006), however, in extreme-ly nutrient limited environments such as wetlands on the ultramafic soils in New Caledonia, Cyperaceae were found to be heavily colonized (Lagrange et al. 2011).
Oligotrophic softwater lakes of northern Europe and North America represent a special habitat with relatively low nutrient content. They are typically inhabited by sub-merged isoetid plant species, including
An increasing evidence shows that plants in N limited environments, specifically arctic wetlands and many om-brotophic peatlands, are capable to utilize soil organic N compounds. This can happen by direct uptake of simple amino acids such as glycine, as documented for several species of Cyperaceae from wetlands spanning the geo-graphical range from the tropics to the arctics (Raab et al. 1999), or through connections with ericoid or ectotrophic mycorrhizae (Jonasson and Shaver 1999).
>
Contribution of N-fixation to N economy of macrophytes
An important contribution to N supply to wetland macrophytes can be from N-fixation (Scott et al. 2007), either by symbiotic N fixers (Elliott et al. 2009) or hetero-trophic N-fixing bacteria living either in the rhizosphere of wetland plants (Dakora and Drake 2000, ?antr??kova et al. 2010) or endophytically (Reinhold-Hurek and Hurek 1998). Diazotrophs of particular importance in wetland and water ecosystems are anaerobic bacteria represented by
While a great deal of research has focused on the mech-anism by which macrophytes survive in nutrient limited environments, the response of macrophytes exposed to ever increasing amounts of nutrients has been receiving more attention relatively recently (Downing et al. 1999). Agriculturally driven eutrophication has had a profound impact on macrophyte abundance and community com-position in shallow lakes. Shifts in macrophytes commu-nities from a dominance of submergent (e.g.,
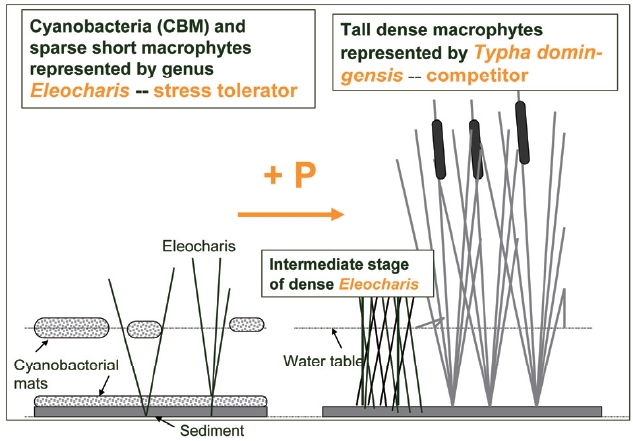
Nutrient enrichment often leads to species replace-ment of slower growing, nutrient saving stress tolerators by rapidly expanding competitors (Fig. 3). Results of mul-tiple studies on ecosystem response of this change from long term nutrient enrichment plots in subtropical/tropi-cal marshes of the Florida Everglades and Belize, Central America document profound impacts of nutrient input on macrophyte and microphyte species composition leading to a switch from an auto- to heterotrophic metabolism in the water column (Hagerthey et al. 2010), replacement of the autotrophic by heterotrophic N-fixation (?antr??kova et al. 2010) and many other changes (see a special issue of Critical Reviews in Environmental Sciences and Technol-ogy, 41[S1], 2011, for more examples and references).
The majority macrophytes rooting in anoxic sediments have developed an aerenchyma, a tissue including large intercellular spaces allowing the transport of oxygen from the atmosphere to roots and rhizomes. The oxygen trans-port enables the continuation of aerobic metabolic pro-cesses in an otherwise reduced environment (Armstrong et al. 1994, Laanbroek 2010). Consumption of oxygen in the belowground plant parts leads to a diffusion flow of air from the shoots to the roots and rhizomes, whereas the metabolically produced carbon dioxide and/or meth-ane follows the opposite route of diffusion (Laanbroek 2010). In many emergent plant species, as well as in some floating-leaved species, this diffusive gas flow is replaced by a more efficient pressurized flow (Dacey 1980, Arm-strong and Armstrong 1991). Pressurized ventilation is induced by the temperature or humidity triggered gra-dient between the air outside the plant and the gases in the plant’s aeration system (Armstrong et al. 1996, Grosse and Frick 1999). Pressurized ventilation has been found in many emergent and floating-leaved macrophytes, such as
Emergent wetland plants play an important role in the emission of methane to the atmosphere (Laanbroek 2010). They provide the carbon necessary for the produc-tion of methane either in form of plant litter or as C-rich root exudates (Strom et al. 2003), and they also facilitate the release of methane through aerenchyma (see above). On the other hand, part of the oxygen transported from the atmosphere through aerenchyma to rhizomes and roots, is released in the root zone where it can contrib-ute to the oxidation of methane. Generally, much higher methane fluxes have been reported from the emergent macrophytes such as
There are conflicting data on the contribution of C-rich root exudates to methane production. Juutinen et al. (2003) found that recent products of photosynthesis of wetland plants made a very limited contribution to meth-ane production, and labile organic carbon for methane production was mainly derived from plant litter. On the other hand, experiments conducted with various rice cul-tivars reported the effect of root exudates on total meth-ane emission (Kerdchoechuen 2005).
Another discrepancy concerns the relationships be-tween macrophyte biomass and methane production. Strom et al. (2005) and Kao-Kniffin et al. (2010) showed a negative correlation between plant biomass and meth-ane emissions. In contrast, other studies have shown positive relationships between biomass, CO2 fixation, or net ecosystem productivity and methane flux (Chanton et al. 1993, Christensen et al. 2000). Furthermore, in an Alaskan wet meadow tundra, plant biomass did not influ-ence methane emissions from two different sedge species (Schimel 1995).
Decomposition is a basic process in ecosystem carbon flux and nutrient cycling (Hoorens et al. 2003), especially so in ecosystems where little of primary production is consumed as a living tissue and most ends up in the detri-tus food chain, where the energy bound in organic matter OM is released in a process of respiration by heterotrophs (Ayyappan et al. 1986). Wetlands are often characterized by low herbivory and, unless impacted by humans, they are often ecosystems with low nutrient input. In these limited nutrient environments, the continued availability of nutrient resources often depends on decomposition of organic material (Shaver and Melillo 1984, Aerts and de Caluwe 1997, Alvarez and Guerrero 2000). Decomposi-tion processes are regulated by three interacting sets of factors: physico-chemical environment, the quality or organic material, and the decomposing organisms (Cou-teaux et al. 1995, Morris and Bradley 1999, Liski et al. 2003, Bunemann et al. 2004). In systems where temperature and moisture are not constraining, the most important determinants of decomposition rates are chemical prop-erties of the decomposing material (litter quality) and site quality, i.e., nutrient availability and decomposer activ-ity at the site where the decomposition occurs (Vitousek 2004). Decomposition rates are then related to litter/site quality indicators such as C:N, C:P, lignin:N and N:P ratios (Aerts and de Caluwe 1997, Rejmankova and Houdkova 2006). Under similar conditions, the soft tissue macro-phytes of submersed, floating-leaved and freely floating categories decompose faster than tall emergents (Kim and Rejmankova 2004). Information about decomposi-tion rates and their dependence on environmental condi-tions is important for estimates of carbon sequestration.
To characterize ecological problems, ecological stoichi-ometry is being used with increasing frequency (Sterner and Elser 2002, Tessier and Raynal 2003). For example, N:P ratios have been applied to identify thresholds of nu-trient limitation in wetlands (Koerselman and Meuleman 1996, Aerts and Chapin 2000). Based on studies of Euro-pean wetland plants, thresholds of foliar N:P ratios were found to be < 14 for N limitation and > 16 for phosphorus (P) limitation. Tissue nutrient signatures correspond with ecophysiological differences between growth strategies and with nutrient conditions present in the habitats to which these growth strategies are best matched (Willby et al. 2001). Tissue nutrient contents thus can be used to predict changes in vegetation and corresponding eco-system processes caused by eutrophication, or to detect such changes at early stages before they become difficult to reverse.
Wetlands are complex environments with spatial and temporal heterogeneity in hydrology and resource sup-ply. They often support complex vegetation mosaics, which provide habitat for diverse communities of organ-isms, both invertebrates and vertebrates (Dvo?ak and Best 1982, Gonzalez Sagrario and Balseiro 2010). Changes in vegetation caused by increases in nutrient supply (often from anthropogenic activities) result in changes in habitat suitability. As an example: in wetland habitats, phospho-rus enriched runoff from agricultural lands and human settlements causes a replacement of sparse macrophyte vegetation (
Wetlands cover ? 6% of the earth’s land area and shal-low waters cover ? 9% of global area yet the proportion of invasive aquatic and wetland plant species is large (30%) (Zedler 2011). It is because aquatic and wetland habitats are especially vulnerable to plant invasions due to high disturbance and often high nutrients that facilitate rapid expansion of invading species. The invaders tend to form monocultures that displace native vegetation and allow few co-occurring plants to persist. Key attributes for suc-cessful invasions are easily dislodged propagules that can establish in novel habitats, clonal growth, broad ecologi-cal tolerance and long-distance dispersal vectors (Ervin et al. 2006).
The most problematic free-floating species are
Examples of invasive emergent macrophytes forming monocultures are giant reed (
The major impact of macrophyte invasive species in-clude the replacement of native species that can lead to potential extinction, changing food webs, and modify-ing the system biogeochemistry (to date, generaliza-tions about the biochemical impacts of invasive species lag behind predictions of their impacts on community composition). The replacement of a native submersed
Wetland macrophytes comprise taxonomically highly diverse group of plants. Their functions in wetland eco-systems impact many processes such as nutrient cycling and foodweb dynamics. Changes in nutrient availabil-ity often result in replacement of low productivity - high species diversity systems with highly productive species monocultures. Quantity and quality of litter and root car-bon exudates impact sediment heterotrophic microbial processes. Changes in nutrient availability also result in changes in nutrient stoichiometry of plant tissues and nutrient resorption. The roles of macrophytes and sedi-ment microorganisms in wetland ecosystems are closely connected and should be studied simultaneously rather than in isolation.