Plants, which naturally hyperaccumulate heavy metals,are the most obvious tools for phytoextraction purposes. Recently, a number of plants with the ability to accumu-late specific heavy metals have been identified, and the biochemical mechanisms relevant to accumulation and defense against heavy metal toxicity have been thor-oughly assessed (Tong et al. 2004, Xu et al. 2009, Han et al. 2010).
The success of phytoremediation technology is largely dependent on the ability of plants to survive in a delete-rious environment and produce a significant amount of dry matter containing elevated levels of contaminants. The contribution of plant?mycorrhiza associations for plant survival in an unfavorable environment is well rec-ognized, particularly in heavy metal polluted soils. The in-fluence of mycorrhizal fungi on plant nutrition is thought to be greater for elements with narrow diffusion zones around plant roots, such as P and heavy metals (Clark and Zeto 2000). Among soil microorganisms, mycorrhizal fungi are the only ones providing a direct link between soil and roots, and can, therefore, be of great importance in phytoremediation-enhancing heavy metal availability and tolerance to plants.
When metals are at toxic concentrations in soil, mycor-rhizal rather than non-mycorrhizal host plants are able to colonize these polluted sites (Shetty et al. 1994). Thus, mycorrhizal colonization may be the key to plant survival in contaminated environments by enhancing metal resis-tance in plants and also by improving essential nutrients uptake (Davies et al. 1996, Filion et al. 1999). Additionally, mycorrhiza enhance the uptake of heavy metals (Gildon and Tinker 1983, Han et al. 2001).
A number of studies have claimed to show that ecto-mycorrhizas alleviate metal toxicity. In these studies, ec-tomycorrhizal fungi (ECM) have been demonstrated to alleviate growth depression in tree seedlings due to the toxic effects of heavy metals (Jentschke et al. 1999).
Therefore, the objectives of this research were to deter-mine if
>
Plant and inoculum preparation
Stem cuttings of (1 cm diameter and 10 cm long) hybrid poplar,
>
Production of mycorrhizal P. alba × glandulosa
Ectomycorrhizal associations between
A subsample of fine roots (0.1 g fresh weight) was cut into 2-3 mm lengths and fixed in 50% ethanol to assess root colonization by mycorrhizal fungi. For the root colo-nization by ECM fungi, all short root tips that crossed the grid lines (15 lines) were counted and ECM infection was scored on dark blue-colored short root tips. The rate of ECM root colonization was expressed as a percentage of the total number of infected short root tips over the total number of short root tips counted. Root tips colonized by
Thirty days after inserting the stem cuttings, Cd was applied every 3 days over a 5-month period. At each Cd application, approximately 200 mL of 0.4 mM CdSO4 so-lution was applied to the containerized plants. The pots were placed in plastic dishes to retain leached nutrients and the CdSO4 solution. Pots were randomized in the greenhouse and moved about every 2-3 weeks through-out a 5-month experimental period to minimize posi-tional effects. During the experimental period, daily mean temperature and relative humidity were 23.1 ± 2.1℃ and 74.3 ± 10.9%, respectively.
>
Photosynthetic and gas exchange measurements
The photosynthetic parameters, such as net photosyn-thetic rate (
>
Biomass and Cd determination
Shoots and roots were carefully removed at harvest (5 months) and thoroughly rinsed twice with distilled water. Leaves, stems, and roots were partitioned and individu-ally placed in paper envelopes. Oven dry weights were obtained after oven drying the tissues at 70℃ to constant weight.
Dried leaves, stems, and roots (0.5 g each) were ground in a grinding mill (Retsch MM200; Retsch, Haan, Ger-many). Cd content in the ground tissue was measured by inductively coupled plasma spectrometer (ICPS-1000IV; Shimadzu, Tokyo, Japan).
>
Antioxidative enzyme activities
Fresh leaves (0.1 g) were homogenized under ice-cold conditions with 5 mL of 50 mM phosphate buffer (pH 7.0), 10 mM ascorbic acid, and 1.0% (w/v) polyvinylpyr-rolidone (Han et al. 2009). The homogenate was centri-fuged at 20,000 ×g for 30 min, and the supernatant was collected for enzyme assays.
Superoxide dismutase (SOD) was assayed based on the inhibition of the reduction in nitro-blue tetrazolium in the presence of xanthine at 530 nm according to the method of Beauchamp and Fridovich (1971). Activity of glutathione reductase (GR) was assayed as described by Carlberg and Mannervik (1985). The assay was conducted in a reaction mixture containing 50 mM phosphate buffer (pH 7.8), 0.1 mM NADPH, 0.5 mM oxidized glutathione (GSSH), and 0.1 mL enzyme extract. The change in A340 was recorded for 5 min after adding the enzyme extract.
Freshly harvested leaves (0.1 g) were homogenized in 1.5 mL cold buffer containing 5% (w/v) 5-sulfosalicylic acid and 6.3 mM DTPA. The homogenized samples were centrifuged (15,000 ×g, 10 min), and 250 μL of extract was mixed with 50 μL of Ellman’s reagent (5,5'-dithiobis-2-nitrobenzoic acid) in 2.5 mL of 0.1 M sodium phosphate buffer (pH 8) containing 1 mM EDTA. Then, the mixtures were incubated at room temperature for 15 min. Absorp-tion was recorded at 412 nm against a blank sample.
The data were statistically analyzed using SAS System for Windows ver. 8.01 (SAS Institute, Cary, NC, USA). Mean values per treatment were compared using a gener-al linear model. Tukey’s HSD tests were performed when significant differences (
>
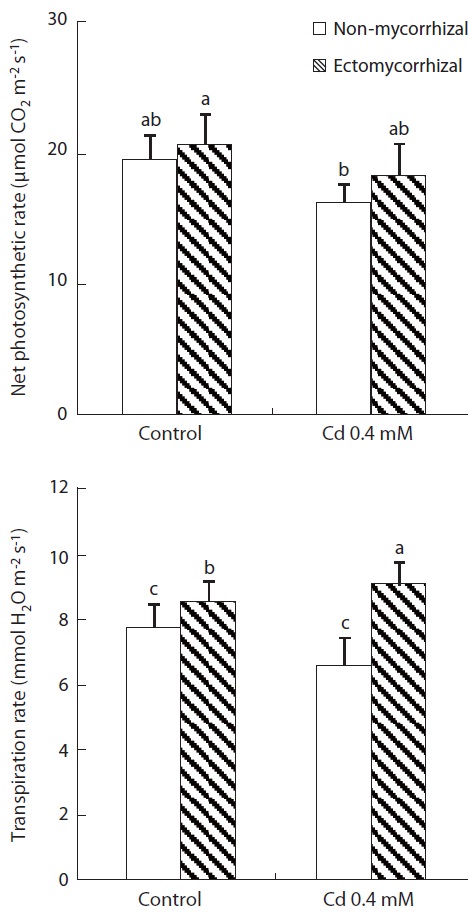
Net photosynthetic rate (PN) and transpiration rate (E)
Both Cd treatment and mycorrhizal infection had ef-fects on
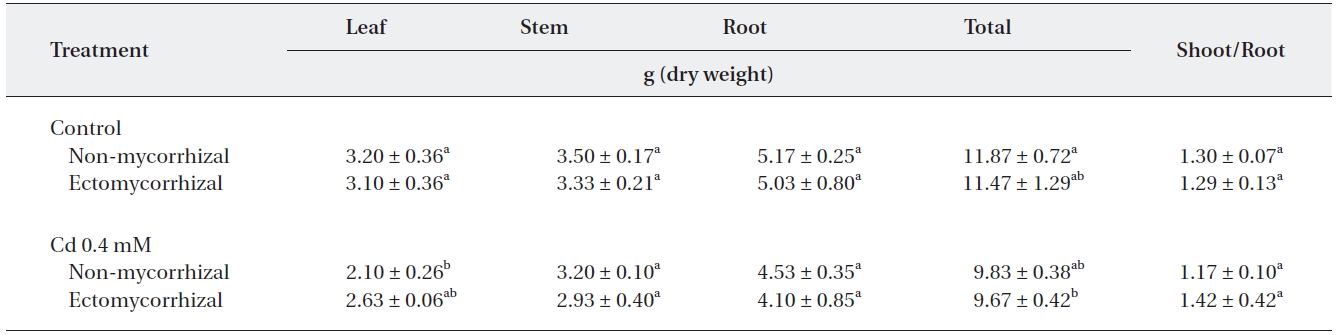
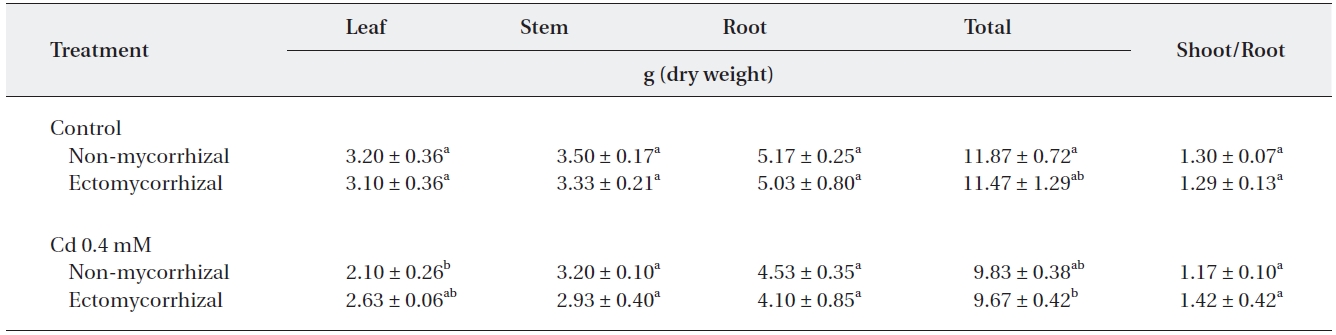
A significant reduction by the Cd treatment appeared only in leaves of NM plants and in total dry weight of ECM plants (Table 1). The greatest reduction in leaf dry weight was observed in NM plants (34.4%) whereas, the greatest reduction in total dry weight was observed in ECM plants. Cd treatment reduced the total dry weight of NM plants by 17.1% and ECM plants by 18.5%, respectively. However, the root to shoot ratio in Cd-treated ECM plants increased by 21.4% over that in NM plants.
>
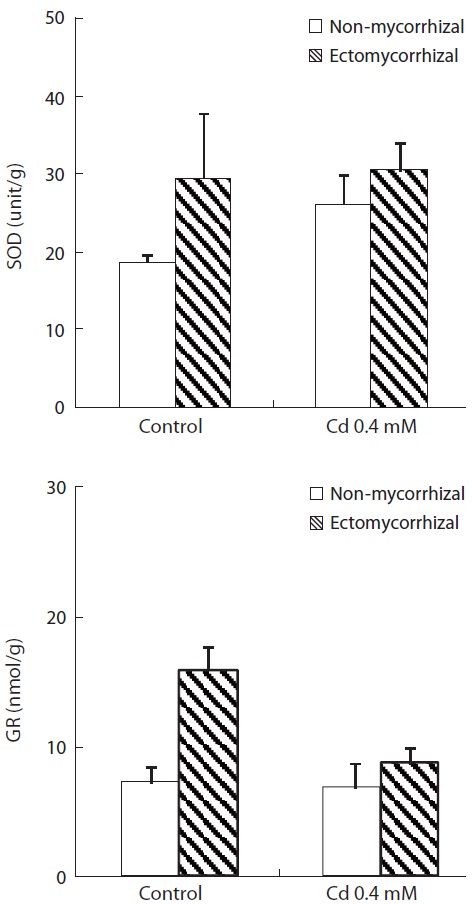
Enzyme activity and thiol content
The enzyme (SOD and GR) activities of plants inoculat-ed with
Changes in dry weight of 6-month-old Populus alba × glandulosa cuttings inoculated with the ectomycorrhizal fungus Pisolithus tinctorius and grown in medium with or without 0.4 mM CdSO4 solution
mycorrhizal infection was greater in control plants than that in Cd-treated plants. More importantly, the increase in GR activity in control plants with a mycorrhizal infec-
tion was 120.8% relative to the NM plants. Cd treatment increased SOD activity and decreased GR activity. The increase in SOD activity by Cd treatment was greater in NM plants (40.3%) than that in ECM plants (3.7%). However, the decrease in GR activity by Cd treatment was greater in ECM plants (45.3%) than that in NM plants (5.6%).
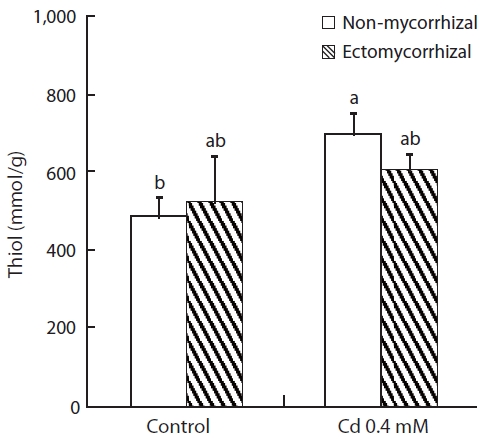
Thiol content increased in both NM and ECM plants treated with Cd solution (Fig.3). The increase in thiol con-tent in NM plants (43.9%) was greater than that of ECM plants (15.6%). No effect of mycorrhizal infection was ob-served for the control or Cd-treated plants.
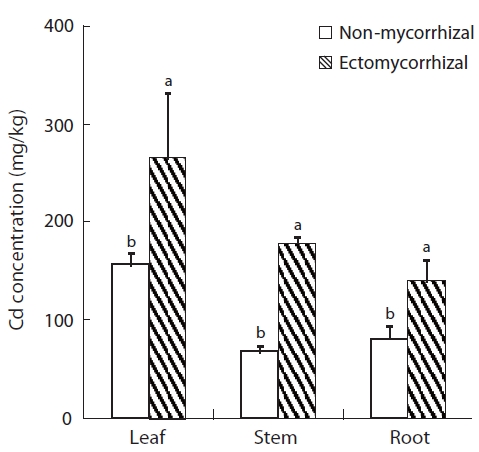
Cd concentration in the leaves, stems, and roots of hybrid poplar increased significantly following the ecto-mycorrhizal inoculation (Fig.4). Cd concentrations in the leaves, stems, and roots of mycorrhizal plants were 69.9%, 167.2%, and 72.8% higher than in the NM plants, respec-tively. Lower Cd concentration was obtained in the leaves, stems, and roots of NM plants (156, 67, and 81 mg/g Cd, respectively) than that in the ECM plants. Cd concentra-tion was highest in the leaves of both NM and ECM plants. The shoot to root Cd concentration ratio was higher in ECM plants than that in NM plants.
Cd is an effective inhibitor of photosynthesis, and heavy metal accumulation in the leaves in higher plants is associated with a reduction in
Mycorrhizal infections alleviate Cd toxicity of their hosts by modifying plant physiological processes such as higher photosynthetic activity. Reid et al. (1983) have shown that the presence of vesicular arbuscular mycor-rhiza (AM) on root systems of plants is correlated with higher
Cd is not a transition metal like Fe and Cu and is not capable of generating reactive oxygen species (ROS) by catalyzing Haber-Weiss or Fenton type reactions (Deckert 2005). Nevertheless, Cd toxicity results from an alteration in the oxidant levels in plants (Foyer and Noctor 2005). Cd
accumulation is correlated with ROS generation in sensi-tive clones of
To scavenge ROS, plants possess a well-organized anti-oxidative defense system comprising enzymatic and non-enzymatic antioxidants. The cooperative function of these antioxidants plays an important role scavenging ROS and maintaining the physiological redox status of organisms (Cho and Seo 2005). Increased SOD activity may be attrib-uted to the increased production of superoxide, resulting in the activation of existing enzyme pools or increased expression of genes encoding SOD (Mishra et al. 2006). Increased SOD activity caused by heavy metals has been previously observed in several plant species and is rou-tinely considered to be an adjustment response to stress (Verma and Dubey 2003). However, we did not observe an increase in SOD activity in mycorrhizal plants (Fig.2). This result might be due to alleviation of Cd toxicity by improving physiological characteristics or a decrease in ROS scavenging ability at high Cd concentrations.
GR is a member of the flavoenzyme family that cata-lyzes the NADPH-dependent reduction of GSSG to gluta-thione (GSH). This reaction is crucial for function of the ascorbate-glutathione cycle and for maintaining a proper GSH/GSSG concentration ratio in cells (Mishra et al. 2006). Increases in GR activity as a consequence of Cd ex-posure have previously been observed in
However, we found that GR activity in the leaves of mycorrhizal plants decreased significantly with Cd treat-ment, suggesting that the drastic decrease in GR activity resulted from other H2O2-scavenging damage by the high concentration of Cd. GR activity in mycorrhizal plants was higher than in NM plants, which may be attributed to an increase in its GSSG substrate similar to that reported by Foyer et al. (1997).
The accumulation of Cd in roots and shoots depends on binding to the extracellular matrix (Horst 1995), com-plexing inside the cell (Cobbett et al. 1998), and transport efficiency (Marchiol et al. 1996). Furthermore, transport efficiency relies on
In this study, the shoots of mycorrhizal plants showed a higher Cd concentration than the corresponding non-inoculated plants, as shown in a previous study (Kelly et al. 1979, Dixon 1988). This result may be attributed to the high concentration of Cd. Under a high Cd concentra-tion, mycorrhizal plants are likely to transport the exces-sive metals in the mycorrhizal roots to shoots. Addition-ally, the increased translocation of metals to shoots may result from the increased
In earlier studies, Davies et al. (2001) and Vivas et al. (2005) demonstrated that mycorrhizal infection under metal-polluted conditions protects against the inhibition of plant growth through enhanced metal tolerance. Sev-eral mechanisms may be involved in enhancing plant Cd tolerance by the coinoculation of microorganisms, e.g., the effect of increasing nutrients (P and K) and in decreas-ing concentration of metals (Cd, Cr, Mn, and Ni). Both mechanisms seem to be responsible for the plant growth effect found in previous studies (Davies et al. 2001, Vivas et al. 2005). However, in the present study,
In conclusion, although mycorrhizal infection under Cd stress ameliorated the physiological characteristics of the host plants to alleviate Cd toxicity through an increase in photosynthetic activity, growth inhibition of host plant was not protected. This result may be due to a decrease in Cd tolerance by excessive Cd accumulation in tissues.