To determine the movement patterns, home range, and use of structural features of captive-bred one- or two-year-old Amur ratsnake (Elaphe schrenckii) juveniles in the natural habitat, we radio-tracked a total of 11 juvenile snakes in a mountain valley in Chiaksan National Park, South Korea, between August 21 and September 20, 2010 and between June 13 and July 13, 2011. During the first week of the release, most juveniles moved short distances, daily, but they increased their distances after the first week. The body weight of the juveniles was negatively related with the movement rate (divid-ing the number of movements by the number of relocations), which was positively related with the mean daily distances moved and the size of both a kernel 50% and 95% home range. During the study period, the juveniles moved daily, ap-proximately 17 m, and the size of the minimum convex polygon and the 50% and 95% kernel home ranges were 1.8 ha, 0.4 ha, and 3.0 ha, respectively. The released captive-bred juveniles were more frequently confirmed underground or on the ground rather than on rocks or on trees. Our results suggest that the body condition of released individuals, the seasonal time of the release, and the existence of available prey and shelters in the habitat should be carefully considered when releasing captive-bred Amur ratsnake juveniles for the rehabilitation of field populations.
Like other animal populations, reptile populations are declining worldwide mainly because of the loss or frag-mentation of available habitats, increased introduction of invasive species, increased spreads of various diseases following climate changes, and continued overexploita-tion of reptiles for pets or medical uses (Mullin and Seigel 2009, Todd et al. 2010). Based on the 2011 IUCN Red List (International Union for Conservation of Nature and Nat-ural Resources 2011), a total of 664 species, which is about 20% of the total reptile species evaluated are assigned as critically endangered, endangered, or vulnerable species, and the numbers are continuously increasing. To delay or reverse this trend, various conservation and rehabilita-tion projects are running (Armstrong and Seddon 2008, Griffiths and Pavajeau 2008).
In particular, because of the decreased population size of endangered species in natural populations, a rehabili-tation project combined with a captive breeding program has been considered a practical method (Gibbons et al.2000, Seddon et al. 2007). In the captive breeding pro-gram, adult reptiles were caught or rescued from field populations for breeding purposes and captive-bred ju-veniles were used for population augmentation or reha-bilitation (Gibbons et al. 2000). To test this method, King et al. (2004) have studied the movement patterns, home ranges, uses of structural features, and hibernation mor-tality of captive-bred eastern massasauga (
The Amur ratsnake (
In this study, we have determined the movement pat-terns, home range, and use of structural features of cap-tive-bred Amur ratsnake (
The pre-adaptation of captive-bred Amur ratsnake juveniles to the study site before release and the radio-tracking study of the released juveniles was conducted at a mountain valley (37°23′ N, 128°02′ E) in Chiaksan Na-tional Park, Wonju, South Korea. We selected this study site for three reasons. First, environmental conditions, such as the existence of a mountain stream, various bask-ing sites, and old dried crop fields in the study site are sim-ilar to those at the place in Woraksan National Park where we studied Amur ratsnakes over the past three years and where we collected adult females to produce the study ju-veniles (Lee 2011). Second, the study site is approximately 400 m apart from our captive breeding facility where we reared the juveniles and where it was known that Amur ratsnakes had been found (Kim 2012). This might be help-ful for the juveniles to easily adapt to the study site. Third, the study site is relatively safe from disturbance by human beings because the area is within the national park. We have expected that these conditions could increase the initial adaptability of the released captive-bred juveniles, probably resulting in a low mortality of them.
There were several rock-cliff areas on the upper parts of the study site, and there were many rock clumps and old tree logs on the floor at the lower parts of the site where Amur ratsnakes mainly were present during the active period (Lee and Park 2011). The mountain stream in the study site was small, and, in several areas, the wa-ter flowed underneath the stream bed. Several pools were separately present within the stream. Since adult ratsnakes sometimes drink and soak in water (Lee 2011), it was thought that this stream may help the released juve-niles to adapt to the study area. Along side of the stream, there were many rock clumps that could be used by the Amur ratsnake juveniles as basking sites or shelters. Veg-etation in the study site was mixed forest. Tree and shrub species included Korean red pine (
>
Juveniles for the study and implanting transmitters
The juveniles used in this study were one- or two-year-old Amur ratsnakes. For the study, we collected eggs in June 2009 from two female Amur ratsnakes (No. 590 and No. 782), which were caught at Woraksan National Park, approximately 55 km apart from the study site; the eggs were artificially hatched, and the juveniles were reared in the captive breeding facility (Kim 2012). In the facility, the juveniles could freely move between indoor and outdoor arenas, and were fed one live or frozen mouse once per week (Kim 2012). We only used the juveniles who success-fully foraged for prey mice, did not have any problems in shedding skin, and had relatively heavy body weight. In 2010, we used five one-year-old juveniles out of twelve, and in 2011, six two-year-old juveniles out of nine. Three juveniles (No. 7821, No. 78213, and No. 78214) were used in both years because appropriate juveniles for the study were limited in 2011. Juvenile No. 7822, used in 2011, was the only female; the others were males (Table 1).
In 2010, we surgically implanted a BD-2 transmit-ter (1.4 g, Holohil Systems Ltd., Carp, ON, Canada) into the abdominal cavity of each juvenile following Lee et al. (2011) and Lee and Park (2011). In 2011, we inserted the antenna of the transmitter under the dorsal skin, but attached the main body of the transmitter on the dorsal skin based on Tozetti and Martins (2007) and Wylie et al. (2011) because we worried about the health conditions of the three juveniles that were used in both 2010 and 2011. The antenna of the transmitter was tightened to the rib of the juveniles. To attach the main body of the transmitter we used glue (Mighty Mendit, Seoul, Korea). The weight of the transmitter averaged 1.5% (range, 1.0 to 2.3%) of the body weight of the juveniles. Following the implantation, the juveniles were allowed to recover for approximately one week in an individual plastic cage (60 cm long × 40 cm wide × 17 cm high) (Lee et al. 2011). The juveniles were moved into the pre-adaptation facility once they had re-covered from the surgery.
>
Pre-adaptation to the natural habitat before release
Before the release of the captive-bred juveniles into the natural habitat, the juveniles were allowed to pre-adapt to the habitat for one week within a pre-adaptation facility (Fig. 1). The facility (180 cm long × 180 cm wide × 150 cm high), which was made of acrylic plates combined with nets, was located at the grassland in the habitat where we later released the juveniles. For efficient air circulation, we made many holes (1 cm diameter) at 10 cm intervals on the acrylic plate walls. We closed the floor and roof of the facility with nets (0.5 cm × 0.5 cm, 1 cm × 1 cm, respective-ly) to prevent the juveniles’ escaping and to protect them from possible predation by birds or mammals. Inside the facility, we placed several tree twigs to provide basking places and also covered some of the twigs using a piece of vinyl sheet (80 × 50 cm) to provide shelters. The water from the mountain stream was provided daily in a plate (10 cm diameter, 3 cm depth). We checked and confirmed the juveniles three times per day (0800, 1200, and 1600 h) and fed the juveniles each one 2- to 3-week-old mouse on
the three days before their release. On the day of the re-lease, we checked the health conditions of each juvenile based on its activity and skin color and also measured the snout-vent length (SVL) and body weight of each juvenile to the nearest 0.1 cm and 0.1 g using a digital vernier cali-per (CD-15CPX; Mitutoyo-Korea, Seoul, Korea) and a digi-tal balance (TMB 120-1; Kern-Korea, Seoul, Korea).
We conducted radio-tracking of the released captive-bred juveniles in random order once per day between 1000 h and 1600 h using a TR-1000 receiver combined with a three element Yagi antenna (Wildlife Materials Inc., Murphysboro, IL, USA) between August 21 and Septem-ber 5, 2010 and between June 13 and July 13, 2011. How-ever, in 2010, the tracking was done once per day or once per two days from September 6 to September 20 because of researchers’ personal schedules.
When we confirmed whereabouts of the juveniles, we recorded the coordinates of the locations using a GPS (Vista CX; Garmin-Korea, Seoul, Korea), determined structural feature types, and, in 2011, checked the state of attached transmitters. Structural features in the habi-tat were classified into four types: underground, ground, rock, and tree. The underground feature was designated if we detected the signal that originated from under the ground or inside rock clumps. The ground feature includ-ed grassland, bare ground, and leafy areas. The rock fea-ture was designated when the snake was found on a rocky
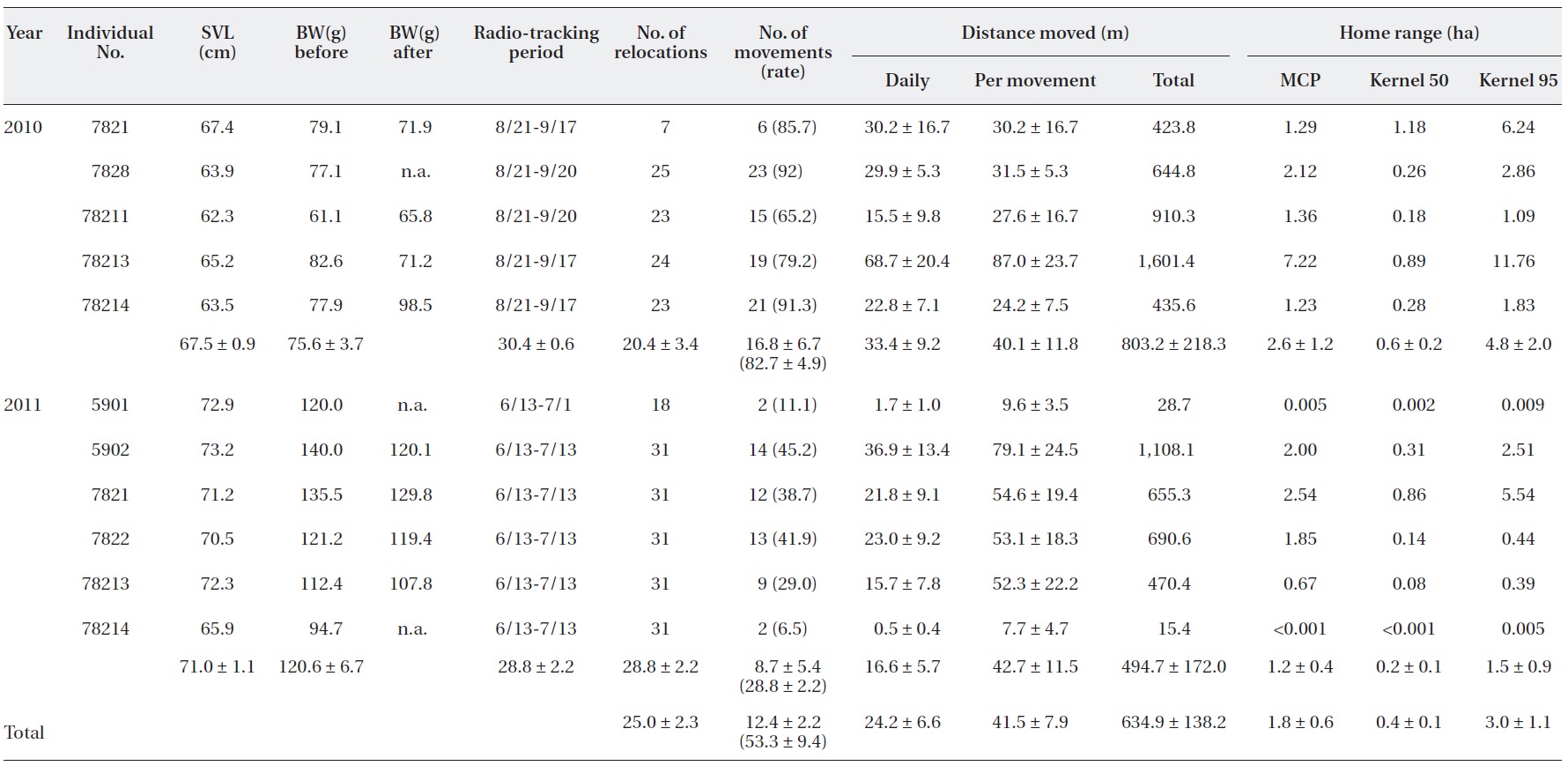
Summary of the radio-tracking of 11 captive-bred Amur ratsnake (Elaphe schrenckii) juveniles in the natural habitat in 2010 and 2011
outcrop or rock. The tree location meant that the snake was observed in a tree above the ground.
We first counted the number of relocations and actual movements of the released captive-bred juveniles. Based on the data, we calculated the movement rate, which was calculated by dividing the number of movements by the number of relocations. We plotted the coordinates of the relocated juveniles on a digital map of the study area in ArcView GIS ver. 3.2 (Environmental Systems Research Institute Inc., Redlands, CA, USA). To calculate the daily distance moved of each juvenile, we only used daily data where two sequential locations were available. Therefore, some data in 2010 where radio-tracking was done once per two days were excluded in this analysis. The daily dis-tance moved was measured as the minimum distance be-tween two locations plotted on the digital habitat maps in ArcView GIS. The mean daily distances moved of each juvenile was obtained by dividing the sum of the daily dis-tance moved by the number of sequential radio-tracking days. The distance moved per movement was calculated by dividing the total distance moved by the number of moved days. The total distance moved was the sum of the daily distances moved during the study period. The home range of each juvenile during the study period was esti-mated using both the minimum convex polygon (MCP) and the 50% and 95% fixed kernel density techniques using the animal movement extension in ArcView GIS following previous studies (Lee and Park 2011, Lee et al. 2011). We selected the MCP home range because MCP does not make any a priori assumptions about the pattern of space use of the lizards, and it is easy to calculate based on real observations of snakes (Haenel et al. 2003). It has been known that the kernel home range might provide information about core habitats (Worton 1989). Daily humidity and air temperature were directly measured at the valley using a HOBO pendant temperature data logger (Onset Co., Onset, MA, USA), and the precipitation data was obtained from the Wonju meteorological center, ap-proximately 11 km away from the study site.
Because of the small sample size, we applied non-parametric statistical analyses. We did not compare the results from the 2010 and 2011 studies; because three juveniles were re-used in the studies, the study period of radio-tracking was different, and the attachment method of radio-transmitters was different between the years. Re-lationships among individual physical conditions (SVL and body weight), the number of relocations, the num-ber of movements, movement rates, moved distances (daily mean, per movement, and total), and home-range sizes (MCP, kernel 50% and 95%) were analyzed using the Spearman correlation analysis. Also, the same analysis was applied for comparison among the mean daily dis-tances moved and weather conditions (mean daily air temperature and humidity and daily precipitation). The difference in the number of relocations of the juveniles at underground, ground, rock, and tree in each study, 2010 and 2011, was separately analyzed using a Chi-square test. When the difference was significant, we applied the same Chi-square test for the post-hoc tests with the Bon-ferroni correction. All statistical analyses were conducted using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation.
We relocated the juveniles at 20 ± 3 times (range, 7 to 25 times;
>
Movement patterns, distances moved, and home-range size
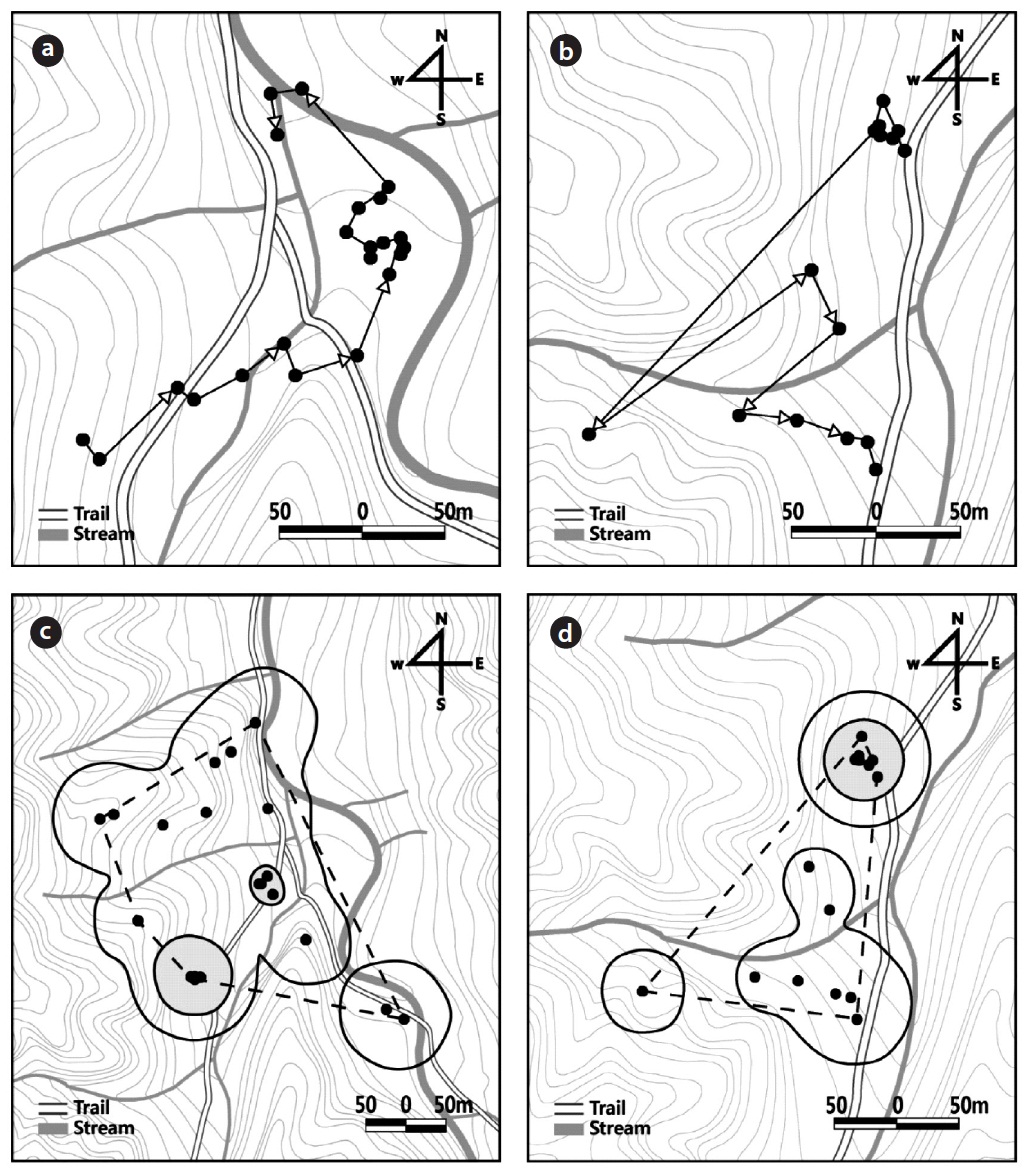
In 2010, the released captive-bred juveniles initially moved to areas at the mouth of the valley and then stayed there. For example, juvenile No. 7828 moved downward toward the stream and was frequently observed on the stream bank or on rocks around the mouth of the moun-tain stream (Fig. 2a). In 2011, the juveniles either moved upward from the release site or stayed for a while at the re-lease site. Juvenile No. 5902, for example, initially moved upward and then moved again farther to the ridge of the mountain (Fig. 2b).
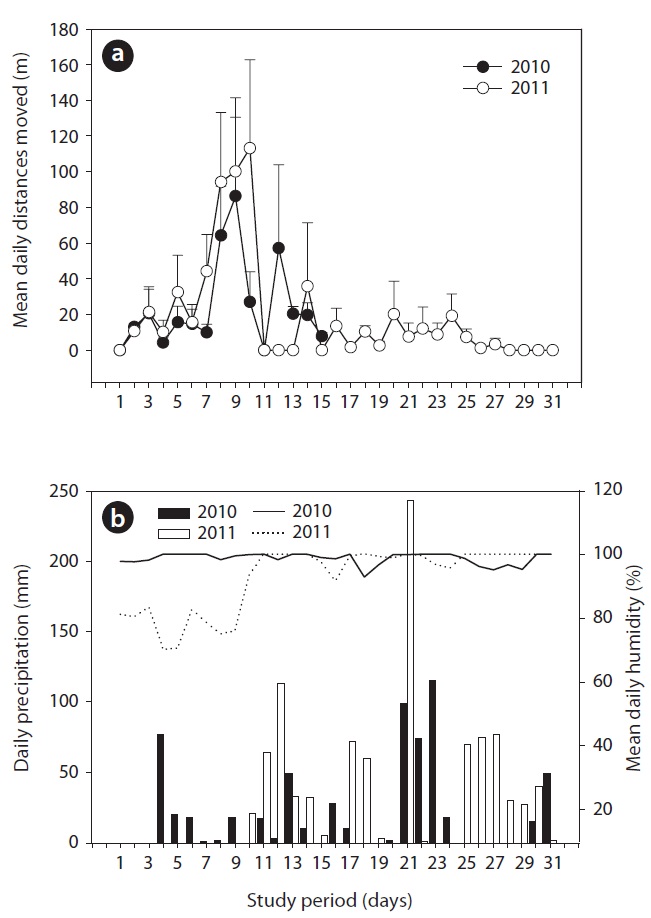
The movement rate was 82.7 ± 4.9% (range, 65.2 to 92%) in 2010 and 28.8 ± 2.2% (range, 6.5 to 45.2%) in 2011 (Table 1). During the first seven days, the released captive-bred juveniles moved short distances, and, after this period, their distances moved increased (Fig.3a). In 2011, from approximately 10 days after the release, the juveniles re-peated their movement patterns and then stayed put. The mean daily humidity (
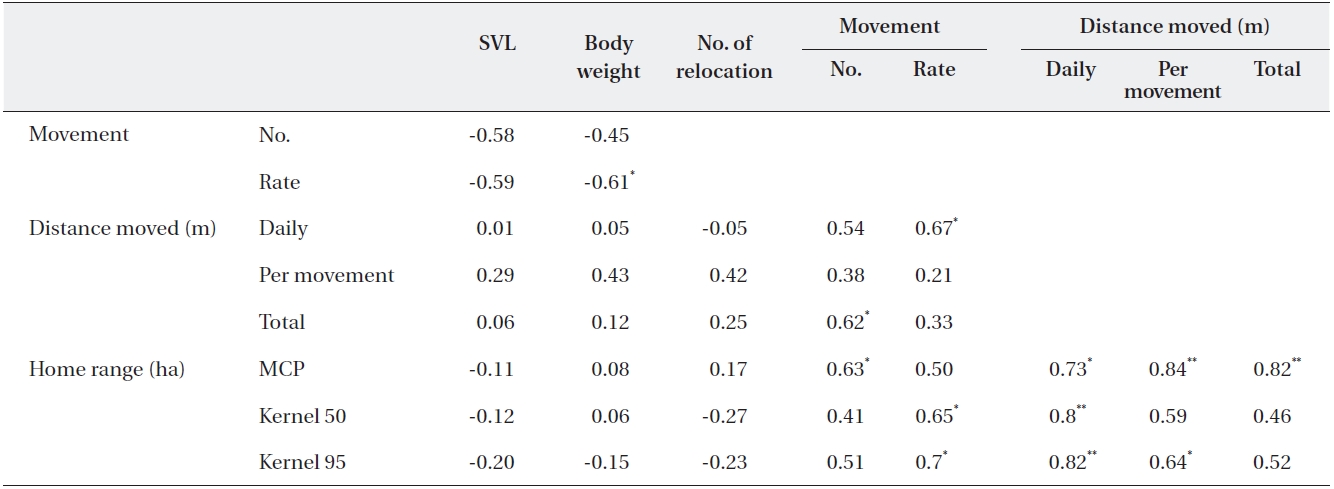
The body weight of the released captive-bred juveniles showed a negative relationship with the movement rate (
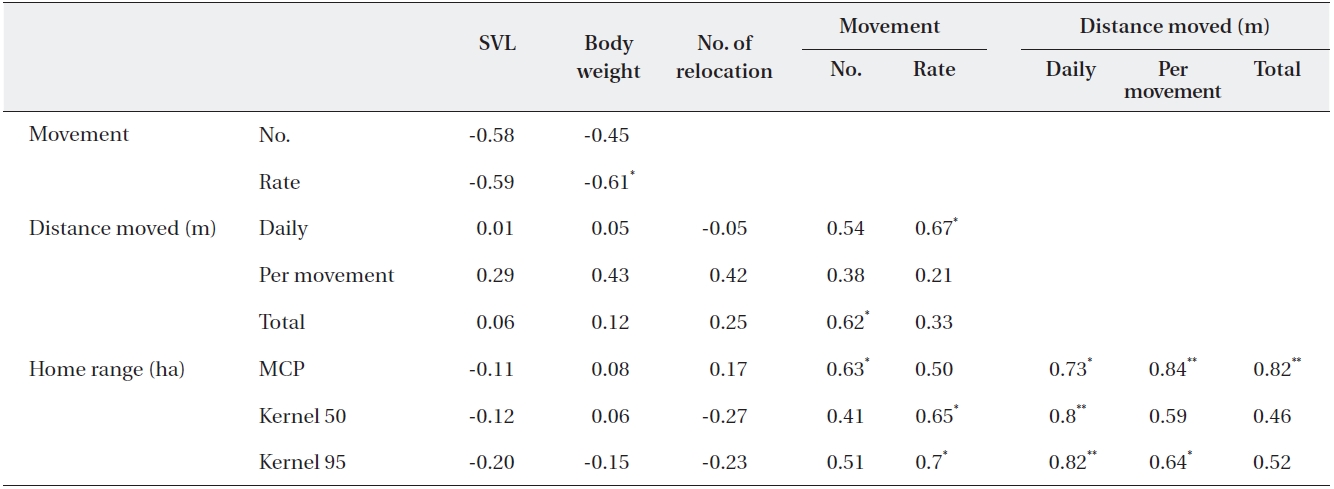
Relationships among the various parameters related with radio-tracking distances moved and home range of captive-bred Amur ratsnake (Elaphe schrenckii) juveniles released into the natural habitat
and 95% home range (
The number of radio-trackings, which was conducted over two subsequent days, was 15 ± 2.8 times (range, 4 to 19 times) in 2010 and 27.8 ± 2.2 times (range, 17 to 30 times) in 2011. In 2010 and 2011, the mean daily distances moved was 33.4 ± 9.2 m, 16.6 ± 5.7 m; the distance moved per movement was 40.1 ± 11.8 m, 42.7 ± 11.5 m; and the total distance moved during the study period was 803.2 ± 218.3 m, 494.7 ± 172.0 m, respectively (Table 1). In 2010 and 2011, the size of the home range of the juveniles dur-ing the study period was 2.6 ± 1.2 ha, 1.2 ± 0.4 ha for the MCP home range; 0.6 ± 0.2 ha, 0.2 ± 0.1 ha for the kernel 50% home range; and 4.8 ± 2.0 ha, 1.5 ± 0.9 ha for the ker-nel 95% home range, respectively (Table 1, Fig. 2c and 2d).
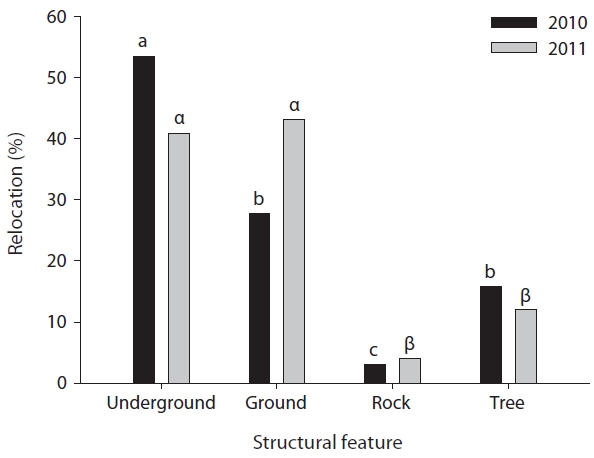
In both 2010 and 2011, the released captive-bred juve-niles were differentially confirmed at the various structur-al feature types: underground, ground, rock, and tree (X2 = 55.5, df = 3,
>
Mortality and changes in the body weight of the juveniles
The mortality of the released Amur ratsnake juveniles during the study period was low. We lost only one juvenile in this study. Three factors might explain such low mor-tality. First, the radio-tracking period was as short as one month. However, this may not be the main factor when considering that in many radio-tracking studies, the mor-tality of released snakes occurred within the first several weeks of their release (Lee and Park 2011, Lee et al. 2011). Second, the activity pattern of the released captive-bred juveniles might decrease the mortality by predation. The juveniles spent quite a lot of time underground, possibly effectively hiding from potential predators. Third, our pre-adaptation stage in the pre-adaptation facility before the release could have been helpful for the habitat adap-tation of the released captive-bred juveniles, considering that such a pre-adaptation process can increase field ad-aptation of captive-bred snakes (Griffith et al. 1989).
In this study, the released captive-bred juveniles did not show successful foraging. Only two snakes out of eight investigated showed some increase in their body weight. Two factors might be responsible for the result. First, the captive-bred juveniles might fail to successfully forage prey in the natural habitat. The feeding program using live or dead mice in the captive breeding facility might not have offered enough opportunity for the juveniles to develop their hunting skills. Moreover, the study habitat where we released the juveniles might not have provided enough available prey for them. Second, during our study period, there was frequent rain. As a result, increased hu-midity and decreased air temperature may have negative-ly affected their basking activity required for proper forag-ing (Huey et al. 1989). The body weight of the recaptured juvenile No. 7828 might have been temporally decreased by the wet weather conditions rather than decreased over the entire one-year period considering its increased SVL.
>
Movement patterns, distances moved, and home-range size
The released captive-bred juveniles did not initially make long-distance movements in the natural habitat, but their moved distances increased starting approxi-mately one week after the release. This movement pattern is similar to previous results (King et al. 2004, Roe et al. 2010). For example, captive-bred juveniles of the north-ern water snake (
The released captive-bred juveniles with heavy body weight moved short distances daily and had small home range. In general, snakes with large body size, longer SVL and heavier body weight, made more movements and had larger home ranges for increased foraging activity (Blouin-Demers et al. 2007). Our results are not consistent with these previous findings. In our study, the released captive-bred juveniles spent quite a lot of time under-ground possibly as a way to adapt to the new habitat or to hide from possible predators. In this situation, the heavy body weight of the juveniles could allow them to stay un-derground for a longer period of time, thus decreasing possible risks from predators. This subsequently could re-sult in short daily moved distances and the use of a small home range. Similar results were recently reported from the study of captive-bred juveniles of the northern water snake (Roe et al. 2010). In the study, large juveniles had relatively small home ranges. These results suggest that by selecting large captive-bred snakes for release into the natural habitat the adaptability of released snakes in the natural habitat may be increased.
The released captive-bred juveniles moved approxi-mately 25 m and used 1.9 ha MCP home range daily during the study period. In a previous study, adult Amur ratsnakes moved daily about 15 m, and, monthly, they used about 1.5 ha MCP home range during the active period (Lee and Park 2011), and the values were slightly smaller than those of the captive-bred juveniles. These results might be because the released captive-bred juve-niles may have actively explored the areas and searched for appropriate microhabitats during the study period.
The released captive-bred juveniles mostly hid under ground and conducted their basking on the ground. In a previous study, adult Amur ratsnakes were most fre-quently observed on rocks or stream banks (> 70% of total observations) (Lee and Park 2011). About 18% were observed on the ground and less than 5% were observed underground. In this study, the released captive-bred ju-veniles were relocated in high-frequency underground (48%) or on the ground (36%), but in low-frequency on the rock (3.5%). Our results suggest that released captive-bred juveniles in the natural habitat use the underground as a hiding place and probably use the ground as a bask-ing place. Previous studies of juvenile snakes also showed that in woodland forests juvenile snakes preferred con-cealed structural features because of their high vulner-ability to potential predators and that they basked on the forest floor. It is reported that the sunlight through forest light gaps could be sufficient for the basking of juvenile snakes in woodland forests (Blouin-Demers et al. 2007). For example, black ratsnake (
Our results provide several important implications for the rehabilitation of the endangered Amur ratsnakes us-ing a captive breeding program. First, the release time of captive-bred juveniles should be carefully selected. In this study, frequent precipitation in June or August negatively affected basking and foraging activity of the juveniles. The release time could be matched to the natural time between late August and mid September (Lee 2011) when hatchlings appear in natural populations. Second, a bet-ter feeding program in a captive breeding program could be developed and the existence of available prey in the natural habitat should be investigated before releasing juveniles. In this study, the body weight of most juveniles decreased, implying that most juveniles may face some foraging problems. Third, considering that the released captive-bred juveniles initially spent quite a long time hiding, the natural habitat where captive-bred juveniles are released should have appropriate shelters and struc-tural features. Also, the habitat should be large enough to accommodate for juveniles’ movement distances and home range.