In this study, we investigated carabid beetles residing in the wetlands to understand their ecological adaptation and strategy selection associated with restricted resources and habitat limitation. The species richness, abundance, seasonal activity, and spatial distribution of the carabid beetles between the Mujechi Wetlands (wetland sites) and Mt. Jeongjok (mountain sites) have been compared. A total of 1,733 individual beetles from 30 species were collected and classified at the studied sites. The wetland sites were identified as having lower species richness and abundance for carabid beetles when compared with the adjacent mountain sites, whereas these beetles were observed to be dominant in the wetland sites than in the adjacent mountain sites. Calosoma inquisitor cyanescens, Carabus sternbergi sternbergi, and Carabus jankowskii jankowskii species were dominant in both the wetland and mountain sites. These species showed significant-ly different seasonal activity patterns in the wetland sites relative to the mountain sites. Although the three listed carabid species were observed to be widely distributed throughout the wetland sites, they still showed preference for drier sites, which clearly shows a distinction in their habitats. The results of the spatial-temporal distribution of carabid beetles in the wetland sites reflect their special strategies regarding space and time partitioning for maintaining their population. The distribution patterns of carabid beetles in the wetland sites also showed the desiccation gradient and environmental changes prevalent in wetlands. Ecological surveys, which use carabid beetles in the wetlands, can then be performed when restoring wetlands and for establishing management practices for improving the habitat quality.
A wetland is an area where the soil is either permanent-ly or intermittently submerged throughout the year, or for varying times of the year, including the growing season. As a result, hydrology determines soil formation and de-velopment (pedology), and in combination, the two vari-ables also affect the structure of the ecological communi-ties present in the wetlands. Because of the dual nature of a wetland, these variables provide conditions that sup-port both aquatic and terrestrial species. The prolonged presence of water creates conditions that favor the growth of specially adapted plants and animals and promotes the development of characteristic wetland soils (Cowardin et al. 1979, Mitsch and Gosselink 1993).
However, because of their characteristic traits, wet-lands create habitat islands in the surrounding landscape matrix, which may or may not be limited in size (areal extent). In typical mountainous areas, wetlands are more isolated and limited in areal extent (Forman 1995), and thus are described as azonal or extrazonal ecosystems rel-ative to the surrounding matrix (Spitzer and Danks 2006). In addition, in wetlands, the climate is cool, precipitation is relatively high, evaporation is often limited, and drain-age may be poor. Hence, for terrestrial species that prefer the dry habitats or are nonadapted to wet conditions, the aforementioned conditions have a negative effect on their hibernation and reproduction (Andersen 2006). In gen-eral, species inhabiting this type of habitats, which have limited resources, could choose special strategies such as temporal, spatial, and food resource partitioning to sur-vive and to maintain their population (Schoener 1974, Niemela 1993).
We investigated the carabid beetles in wetlands for explaining their survival strategies in wetlands that have restricted resources and limited habitat conditions. The carabid beetles show sufficient variation taxonomically and ecologically, and they are too abundant and sensi-tive to environmental changes to be considered a reli-able monitoring group. Specifically, carabid beetles have been observed to be highly sensitive to wetland types and wetland environmental characteristics (Do et al. 2007b). Their habitat distribution, annual and daily activity pat-terns, and diet are determined by abiotic factors (e.g., light, moisture, habitat structure) and biotic factors (e.g., competition, food supply) (Williams 1959, Thiele 1977, Loreau 1985, Kang et al. 2009, Do et al. 2011, Jung et al. 2011).
We tested the following predictions: 1) There is a sig-nificant difference in the assemblage of carabid beetles in the wetlands when compared with adjacent habitats (e.g., mountain), regarding species richness and abundance. 2) Carabid species that preferred the dry habitats limit their spatial distribution depending on the wet condition of the wetlands. 3) Carabid beetles inhabiting wetlands have different seasonal activities when compared with other habitats to survive and to maintain their population. This article also discusses the wetland environmental changes using the carabid assemblages as ecological indicators, for the establishment of wetland management regimes.
We studied the seasonal activity and spatial distribution patterns of carabid beetles at the Mujechi Wetlands (wet-land sites) of Ulsan Metropolitan City (Republic of Korea) and Mt. Jeongjok (mountain sites). The Mujechi Wetlands (35°27′51.13″ N, 129°08′38.94″ E) is a 1.5 ha montane peat bog at 520-530 m altitude on Mt. Jeongjok (700 m above sea level), which was designated as a National Wetland Conservation Area by the Republic of Korea in 1997. The wetlands originated from differential weathering and ero-sion, and it has existed since 1,785 ± 120 y BP (Choi 1998). The annual average temperature and precipitation were approximately 13.8℃ and 1,275 mm, respectively, as re-
corded at the Ulsan Weather Station, 19.4 km away from the Mujechi Wetlands. However, because the weather station is at an elevation of 35 m, it is likely that the ac-tual temperature of the wetlands is lower. Water flows in the wetlands from the south-southwest (SSW) to north-northeast (NNE) direction. The mean groundwater level was -34.7 m, which became shallower with increasing rainfall and deeper with less rainfall (Lee and Kim 2002).
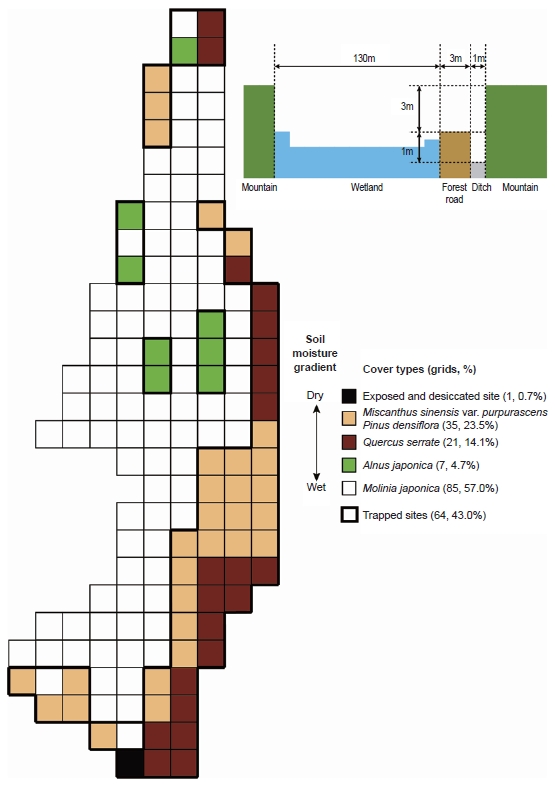
An equal grid map (15 × 15 m quadrats) with simple vegetation, identifying the characteristic vegetation of the Mujechi Wetlands, was used to confirm the sampling sites and environmental variables (Kim and Kim 2003). The dominant wetland plant species are
In the wetlands,
Out of a total of 149 quadrats in the wetlands, pitfall traps (plastic cups, 7 cm diameter) were installed in 64 of them (43.0%). Installing pitfall traps in wetland-grass sites is very inconvenient because of the high water level and the protected status of the
The carabid species richness was compared between treatments using individual-based rarefaction curves. This technique is based on a random resampling of the pool of captured individuals, and it is used to estimate expected richness at lower sample sizes (Magurran 1988, Gotelli and Colwell 2001). The distribution of the domi-nant carabid species and large-sized species that inhabit the wetlands were marked on an equal grid map, which designated where the individuals from each species were caught.
The difference in species composition and seasonal ac-tivities of carabid beetles between the wetlands and the mountain were tested for statistical significance using a paired
>
Diversity and assemblages of carabid beetles
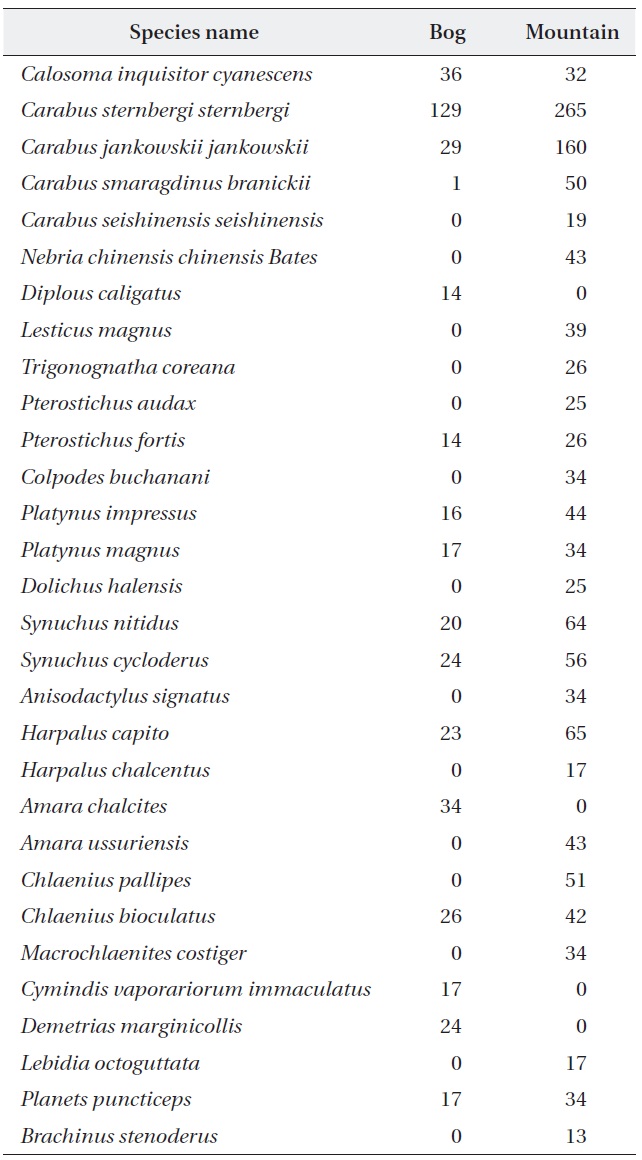
A total of 1,733 individual beetles representing 30 ca-rabid beetle species (including brachinid species) were collected from the studied sites (Table 1). The wetland sites had 16 species (53.33% of total species richness), and mountain sites had 26 species (86.66% of total species richness), respectively. The rarefied species richness was observed to be higher for the mountain sites when com-pared with the wetland sites (Fig. 2). Moreover, carabid abundances between the wetland and the mountain sites
[Table 1.] Carabid beetle species list for the wetland and mountain areas
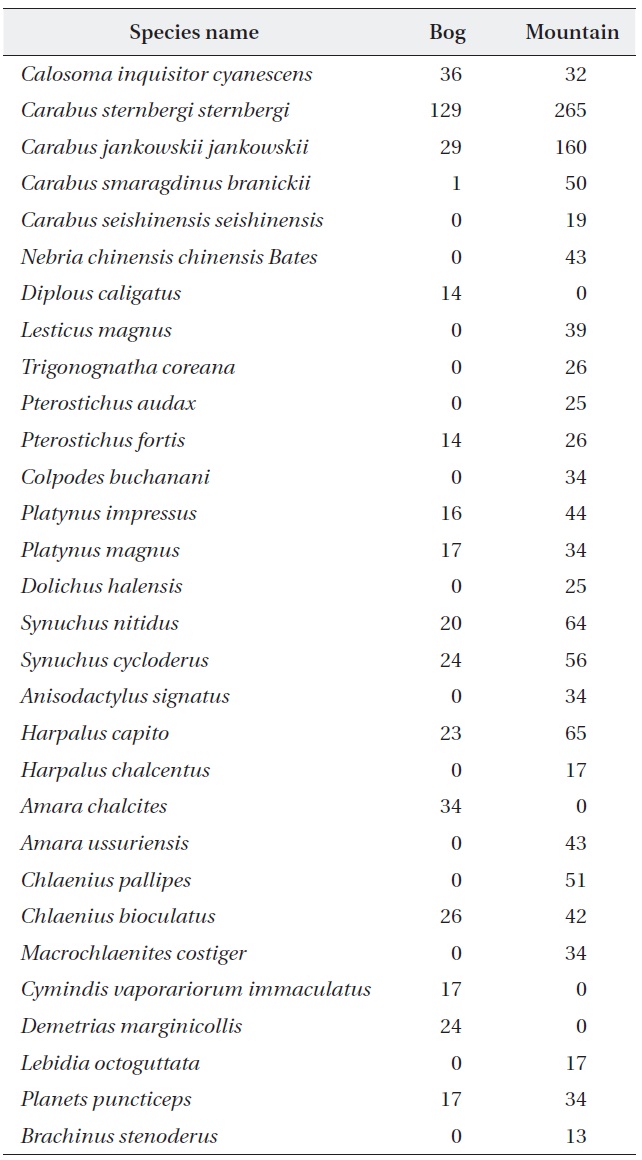
Carabid beetle species list for the wetland and mountain areas
were significantly different (paired
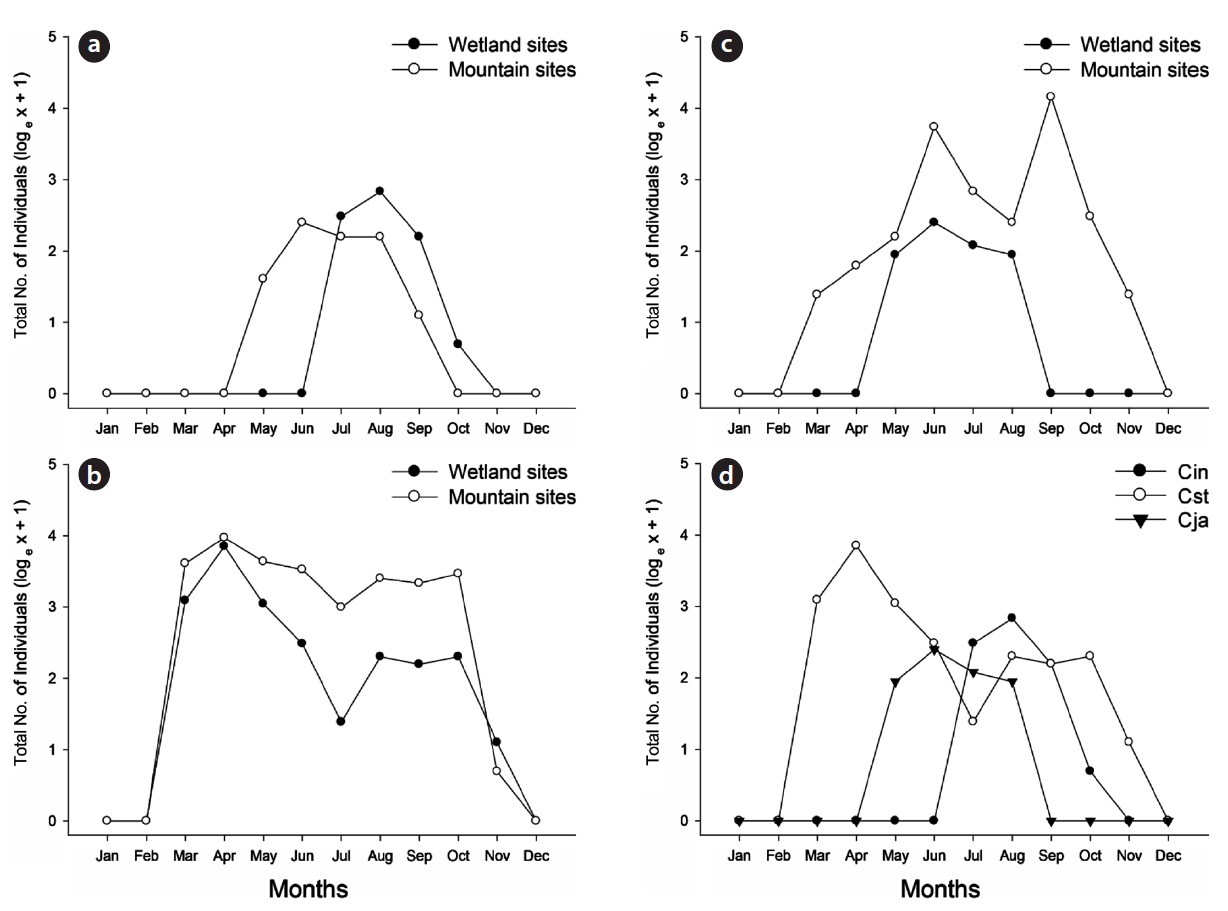
Both the wetland and mountain sites were dominated by
In this study, we investigated
The main activity period of
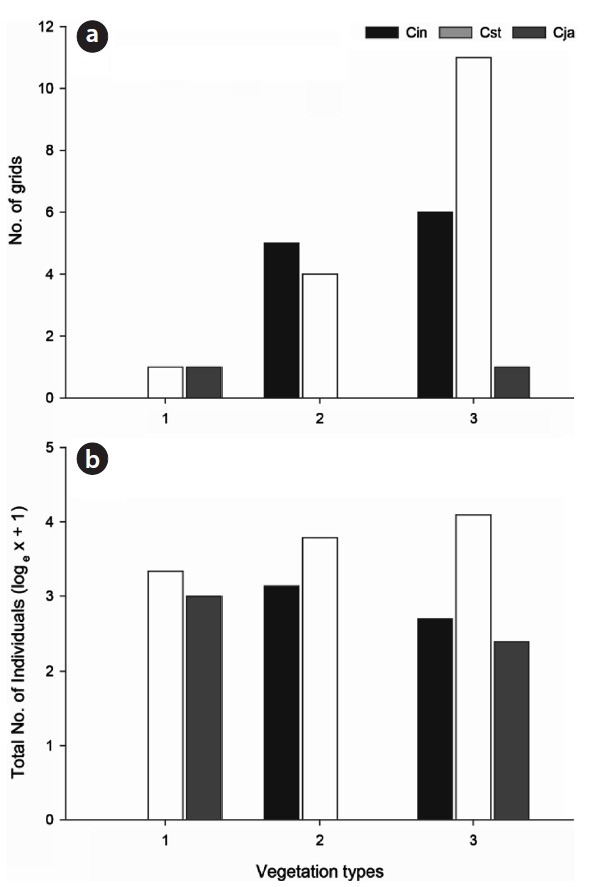
In the wetland sites, the three studied species had significantly different seasonal patterns (
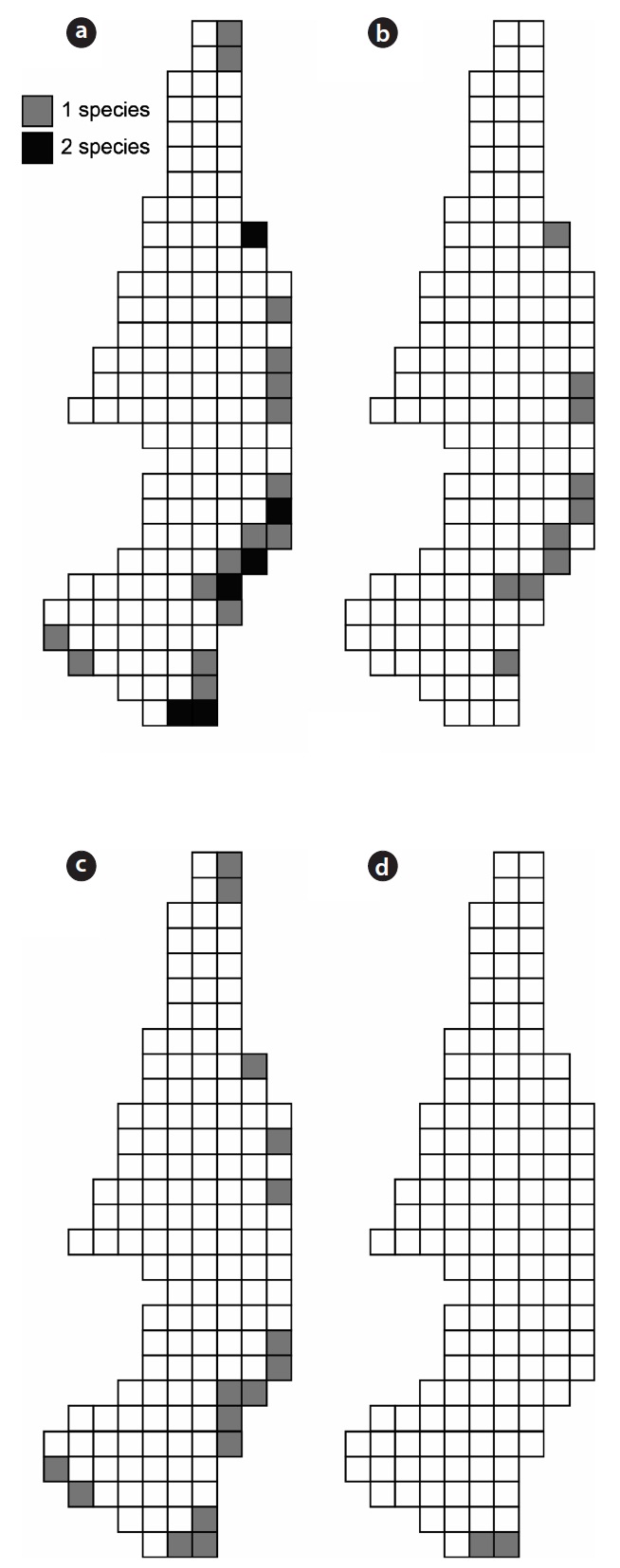
The three species of carabid beetles were present in 22 quadrats (30.99% of investigated quadrats, 12.86% of to-tal quadrats) in the wetlands (Fig. 4). They occurred in 14 quadrats of
We showed that the ecological characteristics of wet-lands affect carabid assemblages. Our results indicate the following: 1) The wetlands have a lower species richness and abundance of carabid beetles when compared with the mountain sites where there are relatively lower num-ber of limiting and disturbance factors. 2) The spatial-temporal distribution of carabid beetles inhabiting wet-land sites was clearly different from those inhabiting the mountain sites. These results show that carabid beetles inhabiting wetlands, where adverse habitat conditions are prevalent, have special strategies (e.g., spatial-tempo-ral partitioning) for maintaining their population.
The carabid species richness and abundance can be determined by the soil and vegetation community struc-ture in wetlands (Brose 2003), especially since soil water contents are a major factor affecting the suitability of a habitat for carabid beetles (Luff et al. 1989, Frambs 1990, Neve 1994). High soil water content is a negative influ-ence during the immature stages of the carabid beetles (Thiele 1977, den Boer 1981). Do et al. (2007a) reported that seasonal flooding of wetlands significantly decreased the species richness and abundance of carabid beetles, although some species adapted and preferred the wet soil condition and were able to recover their population after flooding. Therefore, Mujechi Wetlands, where high soil water contents are present, have lower species richness and abundance of carabid beetles than the mountain sites.
In this article, the seasonal activities of the three cara-bid species (
Although the three carabid species were widely distrib-uted in the Mujechi Wetlands, they showed greater con-centration in the drier vegetation areas. It is proposed that the wet habitat acted as an obstacle to the movement of large-sized species, and hence carabid beetles moved lin-early along the relatively dry edges. It was demonstrated by Riecken and Raths (1996), who studied movement pat-terns using the telemetry method (radio tag), that cara-bid beetles inhabiting wetlands moved linearly because moist sites disturb the movement and diffusion of carabid beetles and drier vegetation types are usually distributed linearly in the wetland edges.
Mujechi Wetlands are undergoing a period of water loss with the soil becoming drier and the wetland thus under-going terrestrialization. Kim et al. (2005) described the distribution and age of alders in the Mujechi Wetlands. The alders extended from the inside to the outside edge of the wetlands and were associated with terrestrialization of the wetlands. However, it is suggested that the distri-bution of the carabid beetles progresses from the outside edge to inside of the wetland on the basis of the desic-cation of the wetland. If terrestrialization of the wetland progresses further, the distribution of the carabid beetles in the wetland can be extended throughout the wetland. Furthermore, when the wetland dries out, the carabid composition in the wetland and the adjacent mountain shows increasing similarity. It will result in a distribution-al range extension, although the carabid species compo-sition of the Mujechi Wetlands was significantly different from the adjacent mountain sites. For improving the wet-land habitat quality, the soil and organic matter migrat-ing from the forest road should be prevented, as it pro-motes the invasion of drier vegetation into the wetlands. In addition, continuous ecological surveys are needed for confirming ecological changes of the wetlands that are as-sociated with anthropogenic and ecological successions.