We analyzed crown development in Aucuba japonica Thunb. var. japonica resulting from the responses of phytoelements to habitat light conditions over a long period of time. Over the years, the degree of extension unit (EU) dimorphism and the degree of anisophylly were higher under shaded conditions than in brighter conditions. An overall temporally increasing pattern in the degree of EU dimorphism was found while no clear-cut trend was found in the case of anisophylly. EU length and number of leaves per EU co-varied in a spatio-temporal context. The number of terminal buds and their sizes acted as the key initiators of morphological differences of phytoelements which were further amplified following bud break. Leaf area density was displayed mostly in the apex peripheral layer of the crown and the apex layer received most of the incident light. There was a tradeoff between annual leaf production and mean leaf size. Depending on the heterogeneity of irradiance level within a crown, correlative growth inhibition caused higher EU mortality at brighter sites. Due to high mortality, shorter EUs had a mere role in the construction of structural framework of the crown except for the formation of some gaps. There was a strong convergence of EU dimorphism, anisophylly, EU extension growth and variations in leaf size towards formation of functional crown to reduce potential self-shading. Depending on the irradiance level, Aucuba japonica Thunb. var. japonica showed two different modes of crown expansion. At the brighter sites, individual crown expansion was progressive while at the darker sites, individual crown expanded in a diminishing manner and maintained a stable size. A plant’s "growth diminishing phase" appeared earlier at shaded sites than brighter sites.
A leaf receives environmental signals and goes under frequent modifications to develop into an adaptive organ size (Goebel 1900, van Volkenburgh 1999, Yano and Terashima 2004) like anisophylly (Ali and Kikuzawa 2005a). Similarly, extension unit (EU)/stem often modifies itself to develop adaptive functionality for example the difference in EU length (Maillette 1982, Ishihara and Kikuzawa 2004). Longer shoot (combination of EU and leaves) specializes in space acquisition while shorter shoot works as gap fillers (Whitney 1976, Halle et al. 1978, Jones and Harper 1987, Yagi and Kikuzawa 1999). All the modifications performed by a plant’s internal and external resource availability (Kume and Ino 2000, Ishihara and Kikuzawa 2009) accrue to develop a functionally adaptive crown.
Establishment and survival of understory plants depend on the efficient exploitation of light resource in the habitat by modification of vegetative crown development, as light is the most dominant limiting factor affecting plant growth (Ali and Kikuzawa 2005b, 2005c). To provide the crown a framework for effective display of photosynthetic organs, plants react to environmental conditions by morphologically changing and arranging modular units such as EU (Niinemets 2010). In view of optimal growth conditions for light interception, a significant relationship between shoot morphology and EU length and leaf size, demography and spacing is to be expected (Ishii and Asano 2010). Variation in above relationships could lead to a different crown shape, even under similar morphological rules of growth (Hiura 1998).
With the likely change in the passage of time habitat light conditions, so do will the plant’s response. To understand adaptation process of a plant, analyses of dynamic modifications of constituents of the crown on a long-term basis are essential (Niinemets 2010). In this paper, we examined whether: i)
Experimental material was
At maturity, multiple EUs extend from a single reproductive bud, which leads to a sudden increase in leaf area in the crown posing severe potential self-shading in the crown (Takenaka et al. 1998, Ali and Kikuzawa 2005c). In the light limited understory conditions, development of efficient canopy to increase light acquisition (Tremmel and Bazzaz 1993) is key to survival. There is a particular dearth of holistic investigation on how developmental changes in phytoelements over years accrue into efficient canopy. Selection of the plant species
We selected ten adult male plants of about 20-25 years of age and plant age was determined by counting the times of branching (Kume and Ino 2000). Each plant had a primary stem that branched on/above the ground and appeared multi-stemmed. All individuals were independent (visual observation) i.e., discrete type (Isobe and Kikuchi 1989).
Various morphological changes in the shoot system initiate in the terminal buds and accelerate further following the bud break. Thus, an understanding of the bud structure is essential for understanding branching pattern, anisophylly etc. and their roles in crown development.
To describe the features of dimorphism of different phytoelements and their integration in a functional crown development, we used various methods to measure the different but inter-related parameters. These methods and parameters are explained below.
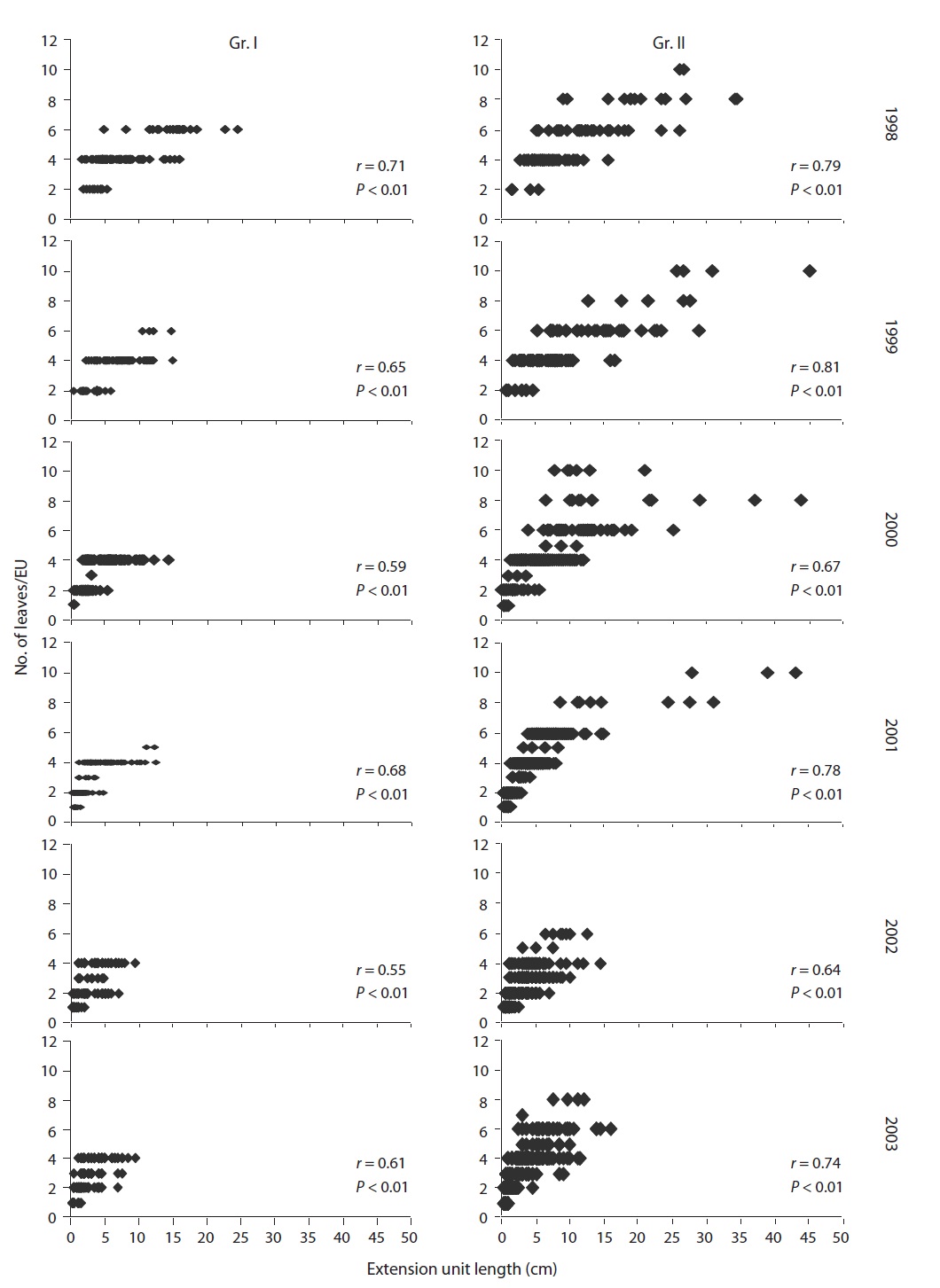
“EU” was defined as the lateral axis that had grown out in a year from the terminal buds (Hara 1980) or sprouted on stems (Ali and Kikuzawa 2005b). This definition was applied to current-year or older EUs (in basipetal direction) with leaves.
>
Measurement of phytoelements in different layers of crown
To identify the layer-wise distribution of phytoelements, their morphological variations and growth dynamics quantitatively, we stratified a crown into horizontal layers of 50 cm depth each. We built vertical columns through the layers of a crown and such sampling column was replicated two to four times at different sites of a crown depending on its size. The randomly selected dimensions of a column on the horizontal plane were 60 cm × 50 cm. As a result, the dimensions of a measuring unit in a layer were as follows: 60 cm × 50 cm × 50 cm (vertical depth of a layer). Within each unit, we measured EU length, counted leaf numbers produced on the EU (fallen leaves were identified by leaf scar on EU), number of EUs per bud and number of dead EUs for the period of 1998-2001. In the case of trespassing boundary of a measuring unit by an EU, we sampled the EU only if nearly 80% of its length (visual observation) was inside the unit, or was otherwise excluded. However, such an event was infrequent.
We measured instantaneous light on each layer along the vertical column by putting sensors (LI-190SA; Li-Cor, Inc., Lincoln, NE, USA) at the center of the column as well as in the open. This measurement was carried out for all replications of the sampling column (two to four times depending on the individual crown size). The difference in photon flux density (PFD) between two consecutive sensors was regarded as light absorbance by the respective layer. Relative photon flux density (RPFD) was estimated in relation to PFD in the open. We measured light between 10:30 am and 2:00 pm standard time on an overcast day around the middle of August.
Additionally, we measured instantaneous light on the sample plants (five to ten locations per crown, depending on the individual crown size) using the same sensors to characterize light conditions on and around the sample plants. After this, RPFD was estimated following the same method described earlier. Irradiance level (on the sample plants) ranged from approximately 0.95% RPFD in closed sites to 8.5% RPFD in relatively open sites.
To compare within and between crowns, specifically the variation in phytoelements, we grouped individuals into two insolation regimes. We formed two groups comprising three individuals each. Four sample plants with intermediate insolation were kept aside to make the groups reasonably discrete. Group one (Gr. I) plants were considered as deeply shaded whose RPFD ranged from 0.95-1.4% while group two (Gr. II) plants were treated as only lightly shaded with varying light levels from 2.5-8.5% RPFD.
We measured laminar length and width (~4,088 leaves) of fully expanded leaves on selected EUs during the period from 1999 to 2003. Then leaf area was estimated by the equation, Y = 0.682 × X (
>
Estimation of distribution leaf area density in the crown
Leaf area density (m2/m3) was estimated as the product of mean leaf size and the number of leaves present in unit crown space.
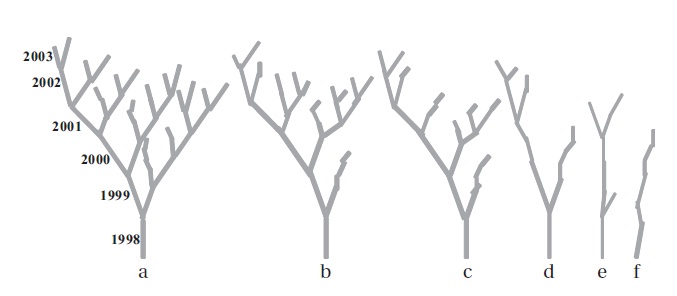
We selected twenty EUs (grown in 1998) per individual in different layers covering the entire crown. On those selected EUs, measurement of the EU length, the number of EUs per bud, the number of leaves per EU, the leaf laminar dimensions and the number of dead EUs were determineduntil November 2003. The entire extension growth (from 1998 to 2003) of a selected EU in the crown was defined as a clump (Fig. 1). The clump signified two important ecological aspects of dynamics of leaf display
structures and pace of space acquisition.
Branching ratio was defined as the ratio of the number of current EUs to the number of one year old EUs. This was applied to compare the branching frequency in different layers of the crown and among the sample plants as well.
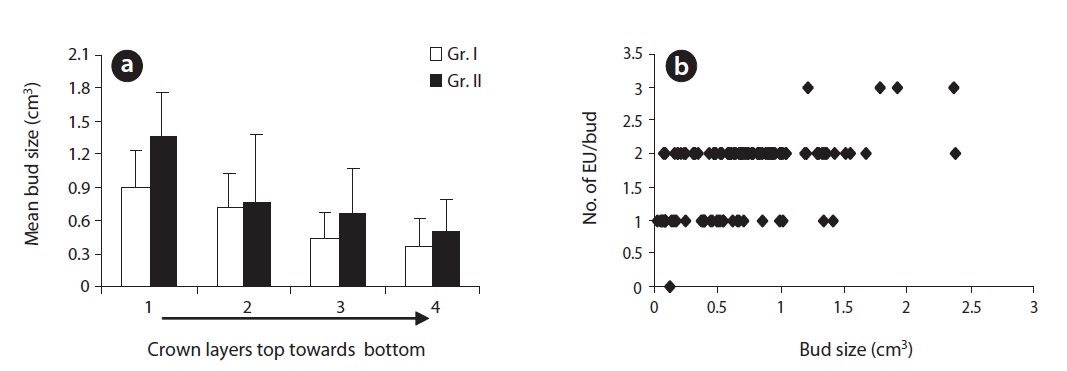
We measured the bud length and diameter of 300 terminal reproductive buds on 300 hundred adult EUs of similar length (30 buds/plant; 15 buds at the apex layer and 15 buds at the lower most layer of the crown). Measurement was done in November (current season) and at the end of March next year before bud break. Buds were rather elliptical, wider in direction of the first pair of bud scales while suppressed in direction of the second pair. Thus, the bud diameter was measured in two directions perpendicular to each other (a, b & a > b) and then averaged. Assuming a conical shape, we estimated the bud size (volume) by the equation,
where, r = radius [estimated as r = D/2 while, D = (a + b)/2] and h = bud length.
>
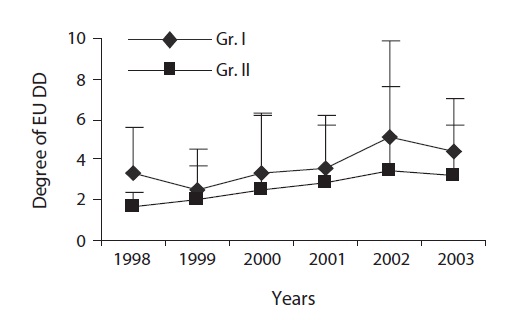
Measurement of the degree of EU dimorphism (DD)
EU is the prime structural unit for leaf display in which the length of EU matters. Length difference among sister EUs (originating from a single bud) initiates in the bud and the difference was furthered followed by bud break. We expressed DD as the ratio of the longer EU (LEU) to the shorter EU (SEU) extending from a single bud. Intermedi-ate EU(s) were excluded in determining DD in which the number of sister EUs exceeded two.
>
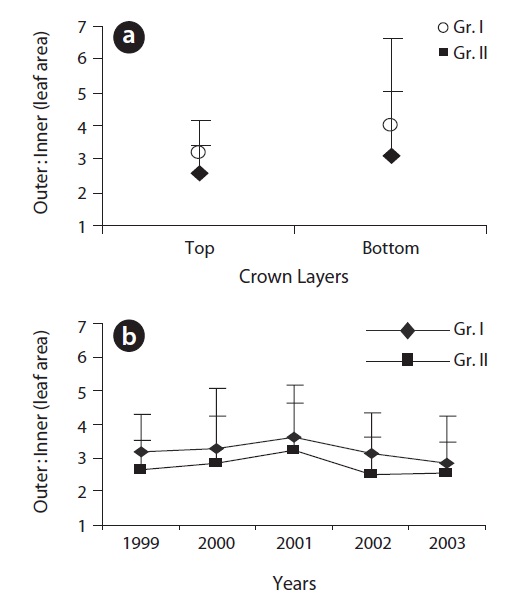
Measurement of the degree of anisophylly
We expressed the degree of anisophylly as the ratio of the leaf area of two leaves opposite to each other on a node. For the anisophyllous nodes, we used the ratio of outer to inner leaf area. The leaf positions were designated in regard to the position of the inflorescence where the outer leaf was positioned away from the inflorescence and the inner was positioned on the side of the inflorescence. The outer leaf was always larger than the inner leaf.
For statistical analyses, Two-way ANOVA, Mann-Whitney U test, and correlation analysis were used wherever applicable, and for obtaining graphs, SPSS Inc. (Chicago, IL, USA) and Excel were used.
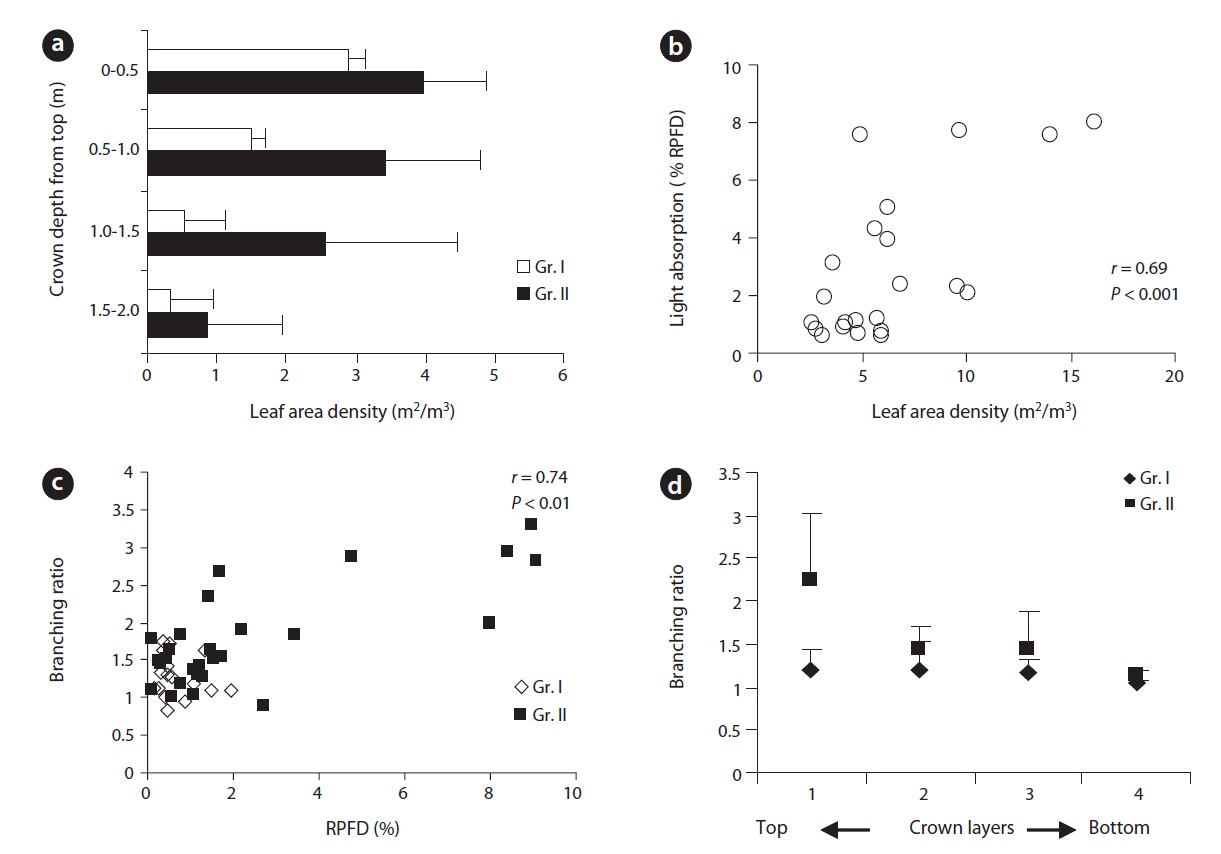
The leaf area of plants was mostly displayed in the upper peripheral layer of the crown and decreased towards the lower layers. Both groups of plants showed similar patterns of leaf area display within the crown. For any comparable layers of the crown, Gr. II plants displayed leaf area density (LAD) twice to that of Gr. I. For Gr. I, there was a significant difference (
A significant positive correlation between incident light and branching ratio (Fig. 2c) indicated that branching ratio was a function of light. The branching ratio was significantly higher in plants (Gr. II) under brighter conditions. Branching ratio decreased from the top towards the bottom of the crown and the difference was significant between the top and the lowest layer of the crown (Fig. 2d). Lower branching ratio led to the development
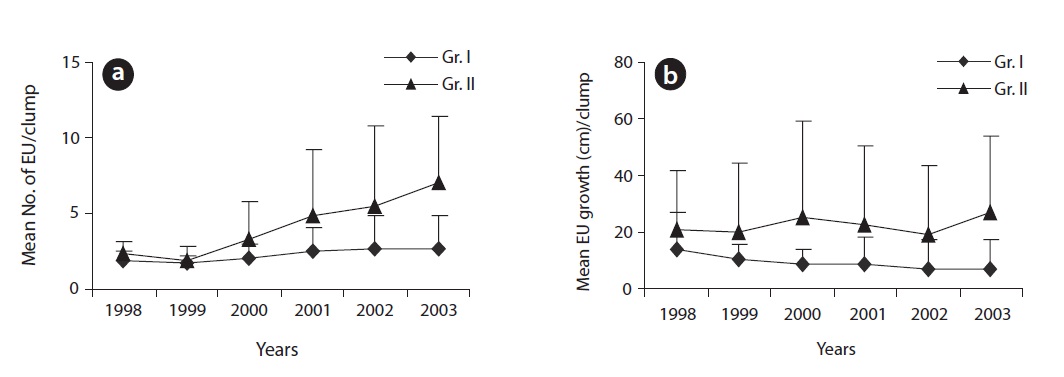
of a sparse crown while the reverse was true for higher branching ratio. The mean number of EUs and the mean extension growth per clump were closer to each other at earlier age of the plants but differed subsequently over time (Fig. 3a and 3b). Incremental mean EU growth (cm) per clump over time indicated expansion of the crown at
a higher rate at brighter sites and diminishing mean EU growth (cm) indicated expansion of the crown at a lower rate at darker sites. Annual structural growth, the sum of EUs, was significantly higher (
Plants (Gr. II) at brighter sites produced significantly larger buds (
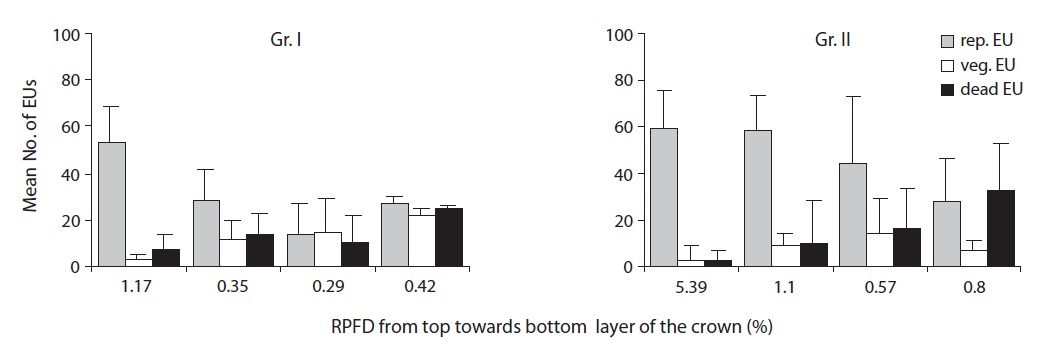
In the top layer of the crown, nearly 60% of EUs were produced from terminal reproductive buds and the proportion gradually decreased down the crown. The proportion of EUs produced from vegetative buds increased towards the lower layers of the crown. Both groups showed a similar pattern of EU production throughout the crown. Gr. I had a greater proportion of EUs produced from vegetative buds compared to Gr. II. EU death showed an increasing trend towards the bottom of the crown. Gr. II had greater EU mortality in the lowest layer than Gr. I (Fig. 5).
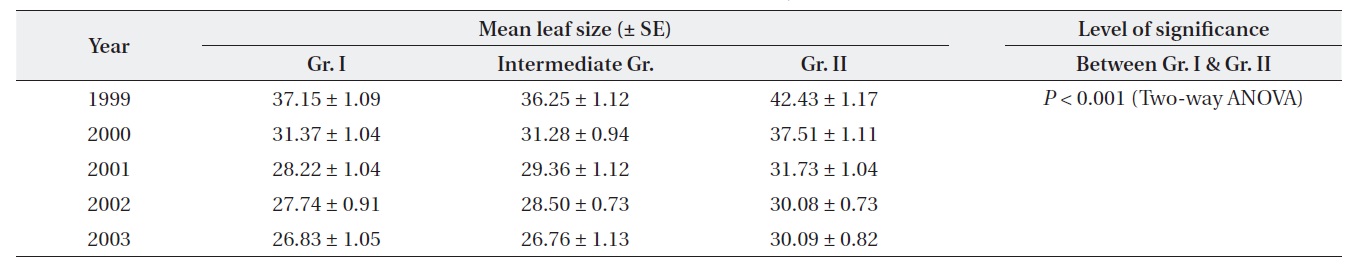
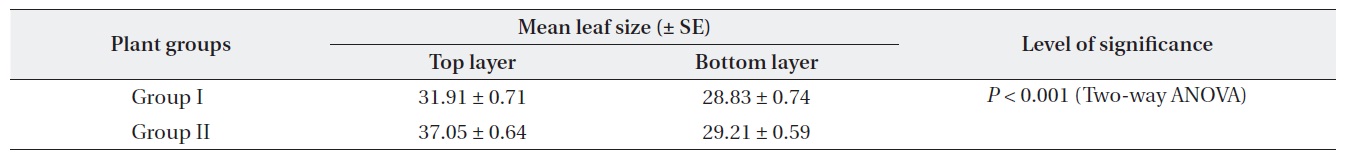
Plants at brighter sites produced significantly larger mean leaf size throughout the period of 1999-2003 (Twoway ANOVA). The mean leaf size gradually decreased over time for both Gr. I and Gr. II as the plants aged (Table 1). For both groups, mean leaf size was significantly different between the top and the lowest layer of the crown. Leaf size difference within the crown was significantly higher for Gr. II than for Gr. I (Table 2).
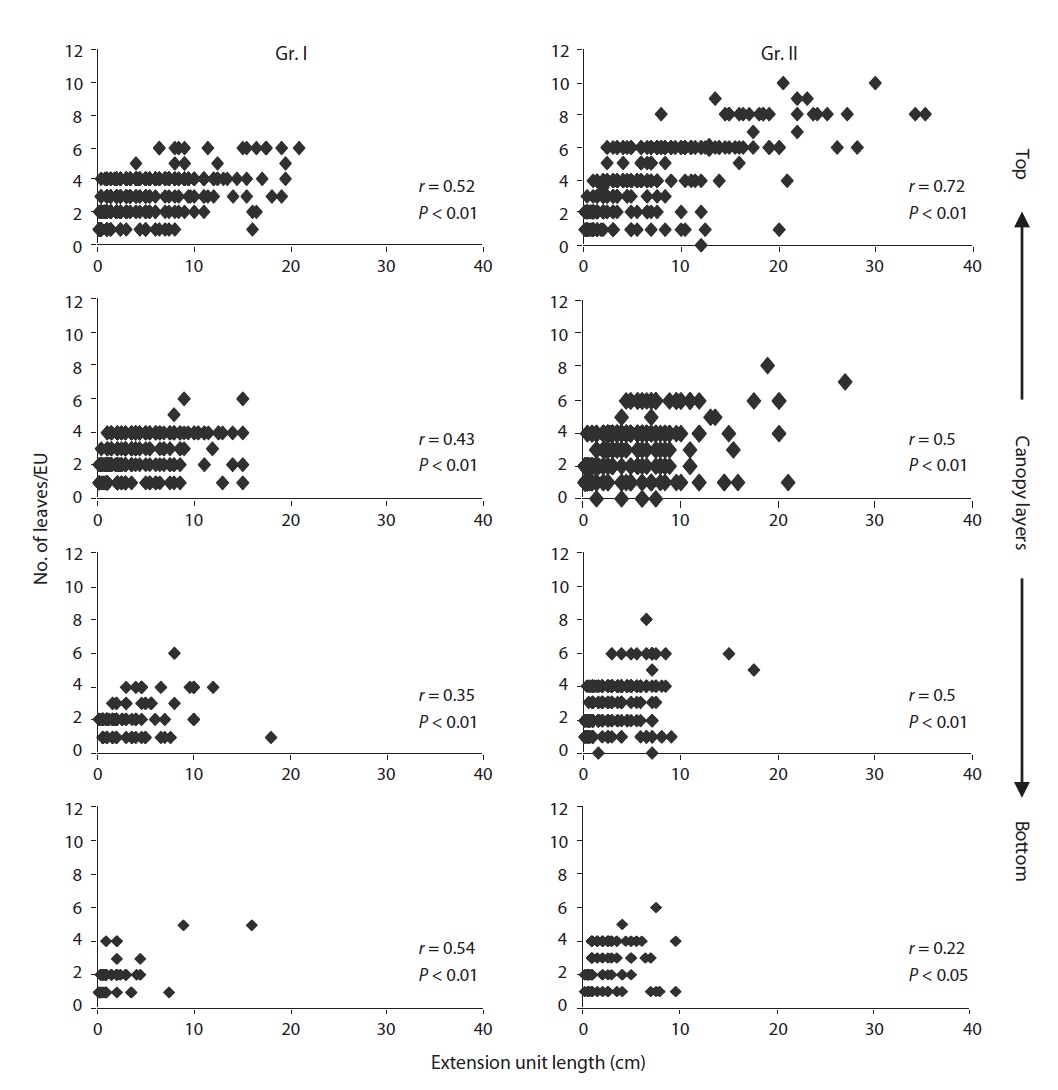
For both groups, there was a positive correlation (
[Table 1.] Differential variation in leaf size (cm2) between the groups of plants in different years
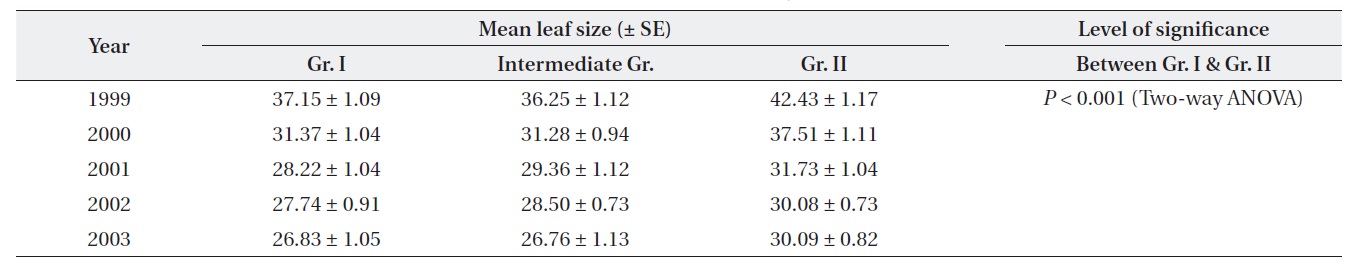
Differential variation in leaf size (cm2) between the groups of plants in different years
[Table 2.] Within crown leaf size (cm2) variation (data pooled from 1999-2003 for each group)
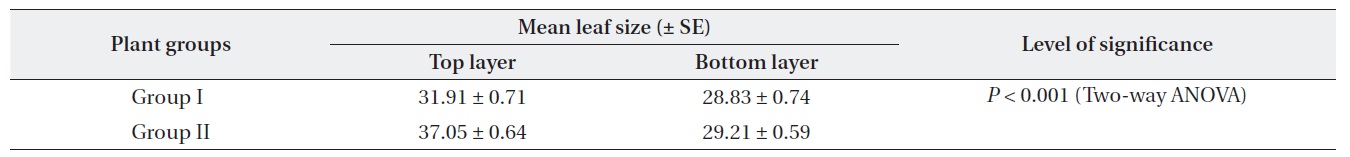
Within crown leaf size (cm2) variation (data pooled from 1999-2003 for each group)
the study. During the study period, the EU length and number of leaves per EU decreased significantly (
The phenomenon of EU dimorphism is accompanied by reproductive maturity and is applicable to EUs extend-
ed from reproductive buds in the mature crown.
The degree of anisophylly was significantly higher at darker sites over the years though year-to-year variation was observed (Fig. 9b).
Structural variations in EUs and their spatial distribution determine foliage distribution in the crown (Ali and Kikuzawa 2005b) and ultimately plant production (Osada et al. 2002, Ishii and Asano 2010) by reducing potential self-shading. Terminal buds, the breeding house of EUs, play a key role in crown formation. At brighter sites and at the top of the crown, terminal buds were mostly reproductive and larger in size leading to a higher branching ratio. They also produced many longer EUs. As a result, extension growth per clump per year (Fig. 3a and 3b) was higher at brighter sites than at darker sites. A higher branching ratio along with larger extension growth provided ample structural support for larger leaf area both in terms of leaf number and individual leaf size (Ali and Kikuzawa 2005b). Due to the positive correlation between leaf area and structural support (Osada et al. 2002), leaf production mostly concentrated in the apex peripheral layer of the crown. Both groups of plants showed a similar pattern of leaf production within a crown. Under darker conditions, plant crowns expanded at a diminishing rate to maintain rather a stable stature. At darker sites, even within a single crown, a significantly lower branching ratio (due to smaller bud size) led to the development of a sparse crown. Contrarily, with a significantly higher branching ratio together with many longer EUs on plants at brighter sites, crowns became denser and expanded at a progressive rate. A higher branching ratio which led to increased EU density especially in the peripheral layer of the crown affected light transmittance through the crown (Ali and Kikuzawa 2005b). However, the gradual decrease in mean leaf size over the years helps plants to minimize self-shading (Osada et al. 2002). Incremental mean EU growth per clump over time indicates expansion of the crown at a higher rate at brighter sites and diminishing mean EU growth indicates expansion of the crown at a lower rate at darker sites (Fig. 3b).
EU dimorphism which helps to reduce self-shading (Yagi and Kikuzawa 1999) initiates in the bud and the extent of dimorphism depends on the size of the terminal bud (Ali and Kikuzawa 2005a). The DD is further amplified following bud break. A significant difference in the DD between the groups indicates the influence of light level on amplification of dimorphism. Increased EU dimorphism (due to differential height growth) i.e., increased difference in length between longer and shorter EUs extended from a single bud enabled leaf display at different heights. Also, it might lead to having openings in the peripheral layer having an enhanced light penetration to leaves on shorter EUs as well as to older leaves in the lower layers, which remain photosynthetically active for a longer time. Greater dimorphism leads a plant to reap more benefit under light deficient conditions where selfshading is critical for survival (Yamada et al. 2000).
Having bi-directional functions (space acquisition and the utilization of acquired space around the base of a longer EU), EU dimorphism has increased the efficiency of the plants (Yagi and Kikuzawa 1999), at least, for the current season. EU dimorphism simultaneously offers discriminate competition between the leader EU and the shorter EU which in turn particularly affects the survival of shorter EUs. With the progress of time and the emergence of new EUs above, older EUs, especially the shorter EUs, moved downward relatively faster and sank deeper in the expanding crown under brighter conditions. At the same time, the photosynthetic capacity of EUs declined depending on the rate and temporal context of the progression from the exterior to interior crown position that varies with the rate of EU growth and leaf turnover (Kikuzawa et al. 2009). In that case, shorter EUs may no longer remain a source of carbon and rather turn into a sink. The smaller EUs invest a significantly greater proportion of their dry mass into leaves and they become smaller with ramifications each succeeding year. As a result, the growth of smaller EUs was suspended and died (Kume and Ino 2000). Subsequently, the role of shorter EUs reduced in terms of photosynthetic gain and crown construction in the long run (Seiwa et al. 2006).
At darker sites or even in the lower layer of a mature crown, many smaller EUs, those which reached below the threshold level for flowering produced a terminal vegetative bud that extended a single smaller EU on the supporting axis (Kume and Ino 2000). Resource constraint prohibited flowering (Ishihara and Kikuzawa 2009) and thus, branching and crown development as well. If the EU size exceeds the threshold level, then flowering/branching could be resumed (Kume and Ino 2000). However, those smaller EUs are often suppressed by other larger EUs (Kume and Ino 2000) that cannot keep pace with crown expansion and die eventually. A denser crown under brighter conditions might cause greater heterogeneity of irradiance levels between the layers of a crown that lead to higher EU mortality due to correlative growth inhibition (Stoll and Schmid 1998, Takenaka 2000). This result caused in turn a higher degradation of the lower part of the crown at brighter sites than at darker sites. The deployment of fewer shorter EUs with fewer smaller leaves in the lower part of the crown together with the higher mortality of EUs accelerated resource translocation to the upper crown to maximize benefit (Ali and Kikuzawa 2005c). Thus, the denser apex layer of the crown developed at the cost of degradation of the lower part of the crown that led to internal recycling of nutrients. Such an internal recycling of nutrients was higher at brighter sites than at darker sites.
The variation in the degree of anisophylly (within and between crowns) was owing to the variation in bud size (Ali and Kikuzawa 2005a). Similarly, the changes in the degree of anisophylly over the years were due to bud size and these bud size variations could be explained, partially, by weather conditions and resource availability over time and space. However, the significance of greater anisophylly under shaded conditions lay in the essence of reducing self-shading since light was the single most limiting factor to plant life (Muraoka et al. 2003), and low levels of self-shading can have a large impact on net photosynthesis near the compensation-point light level (Givnish 1984). The problem of potential self-shading in the crown was resolved through the synchronization of increased EU dimorphism and anisophylly, and a gradual decrease in EU length and by making leaf size smaller over time. The decrease in mean EU length and individual leaf size and number of leaves per EU over the years well indicated the convergence of structural support and the leaf area towards efficient and functional crown development (Ishii and Asano 2010).
The morphology of phytoelements changed in a manner responsive to habitat light conditions and responses varied in a spatio-temporal context. There was significant integration of EU dimorphism, anisophylly, EU extension growth and leaf size variations in the crown to reduce potential self-shading. Shorter EUs had a mere role in crown construction but their death could cause the formation of gaps that were previously filled by them. EU dimorphism that caused discriminating competition among EUs, led to higher EU mortality and paved the way for resource translocation from darker to brighter sites of the crown. Terminal buds appeared as a key initiator of various changes in the phytoelements, which were amplified following bud break while bud types and their sizes acted as the function of light.
Long-term analyses of crown development revealed two different modes of crown expansion. At brighter sites, plant crown expansion was progressive while at darker sites, the crown expanded in a regressive manner and maintained a stable size. A plant’s “diminishing growth phase” appeared earlier at shaded sites than at brighter sites.