Increased atmospheric CO2 concentrations associated with increasing temperatures can directly affect the growth and development of plants through the influence of their physiology. Thus, understanding the interactive effects of elevated CO2 and temperature on plant physiological responses is essential in order for accurate predictions of the effects of climate change to be made.
Much research over the past few decades has focused on the effects of rising atmospheric CO2 concentration and temperature on the physiology of woody plants (Zhou et al. 2011). In general, the short-term exposure of plants to high CO2 levels causes a significant increase in photosynthetic rates and decreases in stomatal conductance (Curtis 1996). However, many studies have shown that accelerated net carbon assimilation is not maintained over longer time periods, and a substantial decline in photosynthesis may occur after long-term exposure to elevated CO2 (DeLucia et al. 1985). Also, stomatal conductance may be increased or decreased, when plants are exposed to elevated CO2 concentrations and temperatures over long periods.
Short-term measurements of the effects of CO2 concentration on physiological responses of plants are not always good indicators of the effects of long-term exposure (Long et al. 2004). Sour orange grown at +300 ppm CO2 above control condition showed that the enhancement ratio for net photosynthesis was about 2.8 in 2 year, but had declined to 1.3 by 14 year, indicating some acclimation to the elevated CO2 (Kimball and Idso 2005), while in a further similar study the photosynthetic rate of loblolly pine was shown to be increased in high CO2 levels during all four years (Tissue et al. 1997).
Woody plants have lower relative growth rates and are less responsive to elevated CO2 and temperature than herbaceous species. Also, woody species may be less functionally plastic to elevated CO2 than non-woody species (Nowak et al. 2004). Thus, it is necessary to conduct long-term experiments on the effects of elevated CO2 and temperature on the physiological responses of woody plants to obtain more significant data.
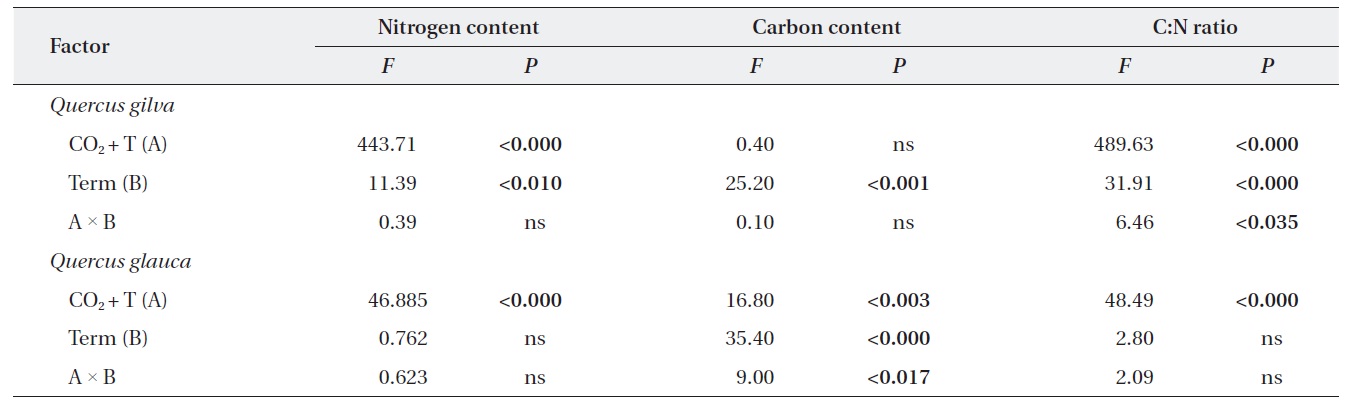
In long-term experiments, down-regulation of photosynthesis is often associated with the responses of leaf chemical compositions, such as leaf nitrogen and carbon contents, to elevated CO2 and temperature (Roumet et al. 2000). In one study, the leaves of seedlings exposed to elevated CO2 had lower concentrations of nitrogen than seedlings exposed to ambient conditions, and nitrogen levels declined by 25% in
The habitat ranges and growth rates of woody species can often be related to temperature (Walther 2002). In particular, evergreen broad-leaved species are considered to be limited by the length of the growing season and absolute minimum temperature (Walther et al. 2001). Peng et al. (2011) determined that if the air temperature increased by 2℃ without changes in precipitation rates, the net primary productivity of evergreen broad-leaved forests would be increased by 5.5%.
The evergreen broad-leaved forest is one of the main vegetation types in warm temperate regions of Korea. It occurs in the region south of 35° N and from 126° to 128° E (Koo et al. 2001). The potential habitats of the evergreen broad-leaved species should expand with increasing temperatures, and hence, it might be suggested that evergreen broad-leaved species could be used as indicators of climate change (Iversen 1944). Thus, for a variety of practical purposes, it is necessary to determine the responses of evergreen broad-leaved plant to elevated CO2 and high temperature.
In Korea, several studies have made on the influence of elevated CO2 and/or higher temperatures on the growth and physiological responses of crops and herbs, such as rice (Kim and You 2010b), meadow-grass (Hwangbo and Kwak 2001), cabbage and radish (Choi et al. 2011) as well as native and invasive plants (Kim and You 2010a, Shin et al. 2012), etc. However, within Korea there has been no such study that tried to determine the ecophysiological responses of evergreen woody species to elevated CO2 and temperature.
We investigated the effects of long-term exposure to elevated CO2 and increased temperature on these two evergreen broad-leaved oaks species, and measured the physiological responses, including photosynthetic rate, stomatal conductance, transpiration rate, water use efficiency (WUE), N and C content and C:N ratio of treatment and control groups. The objective of this study was to understand the long-term responses of
>
CO2 treatments and experimental plants
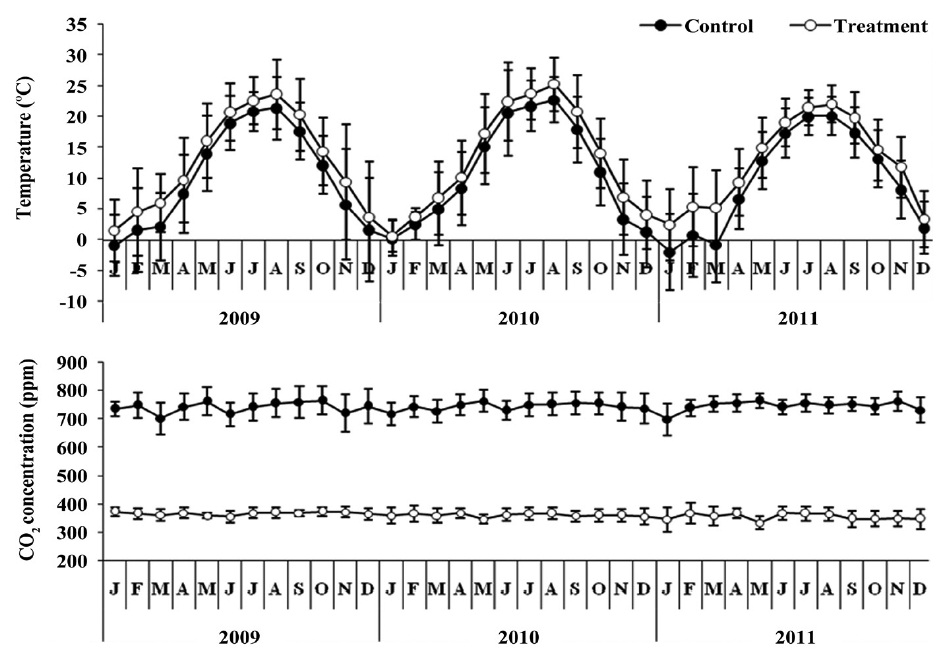
This study was conducted in glass greenhouse (12 m length × 7.8 m width × 5 m height). The surface area of control and treatment is 46.8 m2 respectively. The control was maintained at ambient CO2 concentration, which averaged approximately 360 ± 9.2 ppm on a 24-h basis (Fig. 1). Treatment with elevated CO2 concentration achieved by inputting a small quantity of pure CO2 through two perforated plastic hoses so as to maintain the concentration at approximately 742.30 ± 16.92 ppm, twice that of the ambient (360.38 ± 9.19 ppm) concentration. CO2 concentration was monitored by a CO2 sensor (TEL-7001; Onset Computer, Bourne, MA, USA) at 30-min intervals, and data was stored on data-logger (HOBO U12; Onset Computer) to evaluate the stability of the CO2 concentration in the treatment. The CO2 treatment lasted from May 2008 to June 2011 (39 months).
The mean temperature in the treatment was about 2.5℃ higher than the control (Fig. 1). The air temperature
was measured using a thermo recorder (TR-71U; T&D Co., Matsumoto, Japan) at the same height in the control and treatment greenhouses during the study period.
In March 2008, the seeds of two species were sown in pots (diameter 22.5 cm, height 27 cm) filled with equal volumes of sand, and we fertilized 0.5% of the sand weight. We subsequently applied organic fertilizer, which contains an ammonium nitrogen content below 170 mg/L and nitrate nitrogen at a concentration of 150-330 mg/L (Monsanto Korea Inc., Seoul, Korea). The plants were watered twice or three times per week to prevent them from suffering from water stress.
In January 2009, four seedlings of each species was transplanted into larger pots (120 cm × 85 cm × 48 cm) containing sand, in order to remove root growth restrictions.
The photosynthetic characteristics of the treatment and control plants of the two species were measured at the vegetative stage, using an LCi Ultra Compact Photosynthesis System (Lci Pro; ADC Bioscientific, Hoddesdon, UK) from 10:00 am to 12:00 pm in June, 2009 and 2011.
Three fully expended leaves from the upper part of the plants, and the current year’s growth, were selected from each of the four individuals. All measurements were replicated more than 10 times per leaf section.
The light source utilized natural light, and photosynthetic active radiation (PAR) was 400-600 μmol PAR m-2 s-1. Relative humidity was 70.79 ± 18.56% in the control greenhouse and 72.04 ± 16.59% in the treatment greenhouse. There were no significant differences of PAR or relative humidity between control and treatment greenhouses.
The determined physiological parameters included photosynthetic rate (μmol m-2 s-1), stomatal conductance (mol m-2 s-1), transpiration rate (mmol m-2 s-1) and WUE (μmol CO2/mmol H2O). WUE was calculated as the ratio between photosynthesis rate and transpiration rate.
>
Leaf C:N ratio, nitrogen and carbon contents
The samples of leaves were dried for 2 days at 65℃ . After the leaves were dried, the samples were pulverized into fine powder with a blender (AKM-369s; Eupa, Seoul, Korea). The nitrogen and carbon contents were determined using an automatic elemental analyzer (Flash EA 1112 series; Thermo Fisher Scientific, Rochester, NY, USA) at the Center for Research Facilities, Chungnam National University. The C:N ratio was calculated as the ratio of carbon content to nitrogen content.
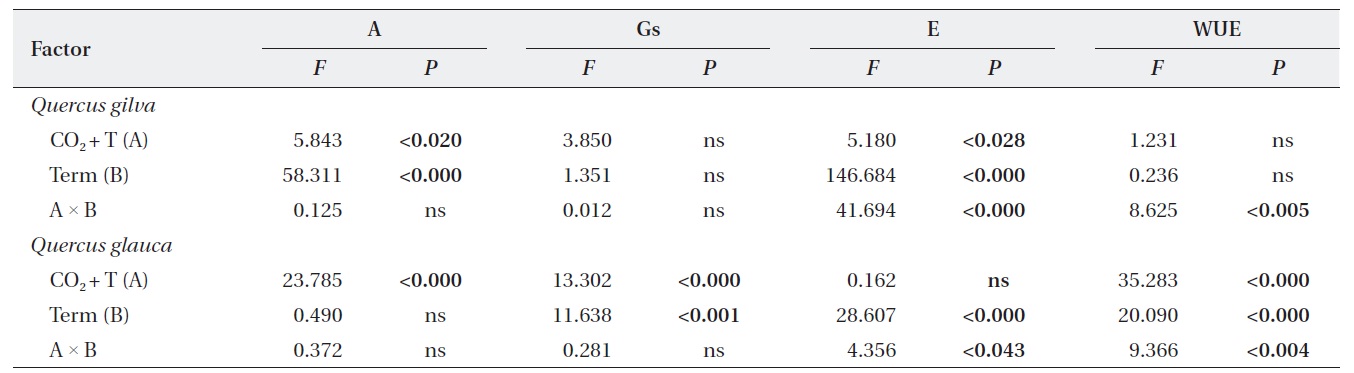
The determined effects of elevated CO2 and temperature on the physiological parameters of two oak species were confirmed via one-way ANOVA, and the statistical
Overall impacts of elevated CO2 temperature and term of CO2 exposure on photosynthetic parameters
differences between the control and treatment groups were evaluated by Fisher’s least significant difference test as post-hocs, with significance set at
All statistical analyses were performed at a 0.05 level of confidence with STATISTICA 8 software (Statsoft, Inc., Tulsa, OK, USA).
>
Effects of elevated CO2 and temperature on photosynthetic responses
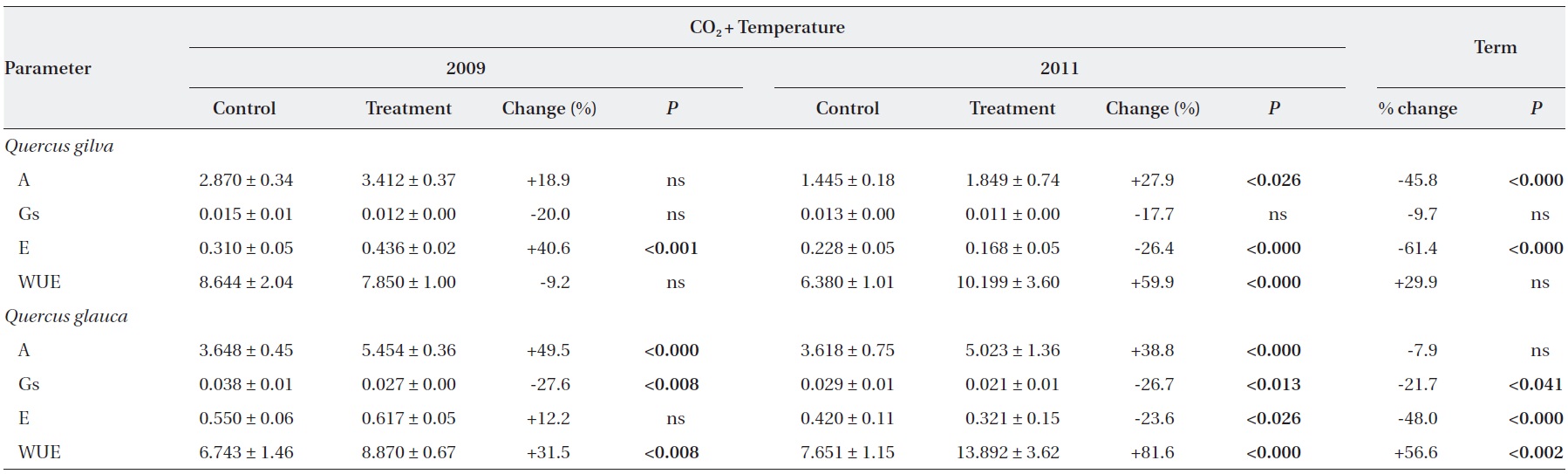
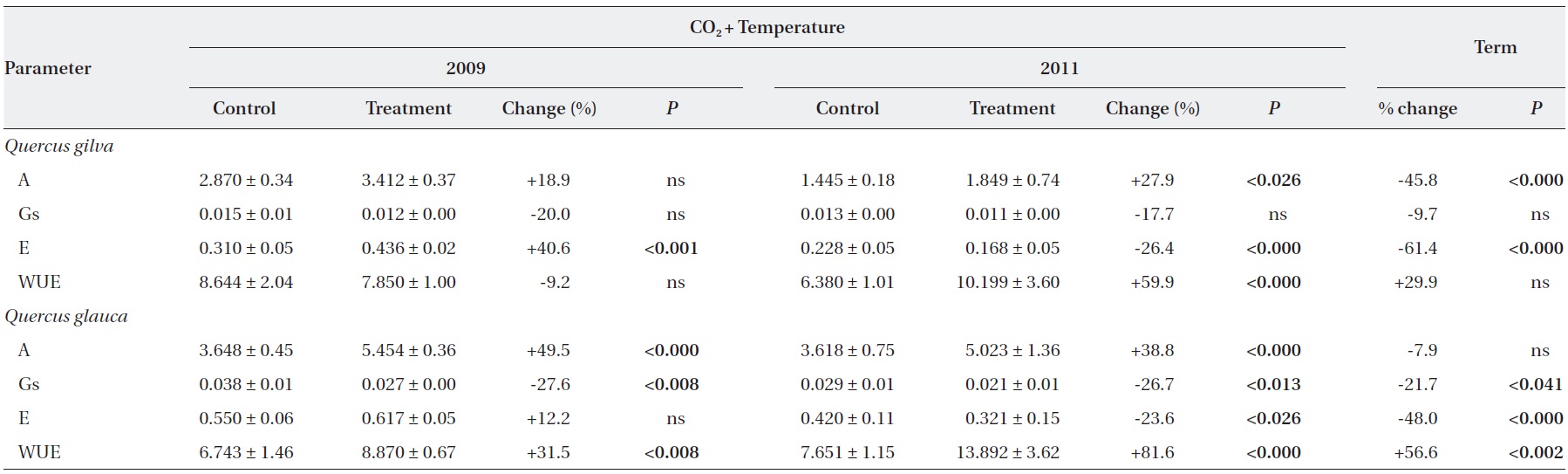
Elevated CO2 and temperature stimulated the photosynthetic rate of both oak species in 2009 and 2011, although the difference in photosynthetic rates of
Stomatal conductance was significantly reduced in
WUE of
>
Effects of terms of CO2 exposure on photosynthetic responses
After long-term exposure to elevated CO2 and temperature, the photosynthetic rate of
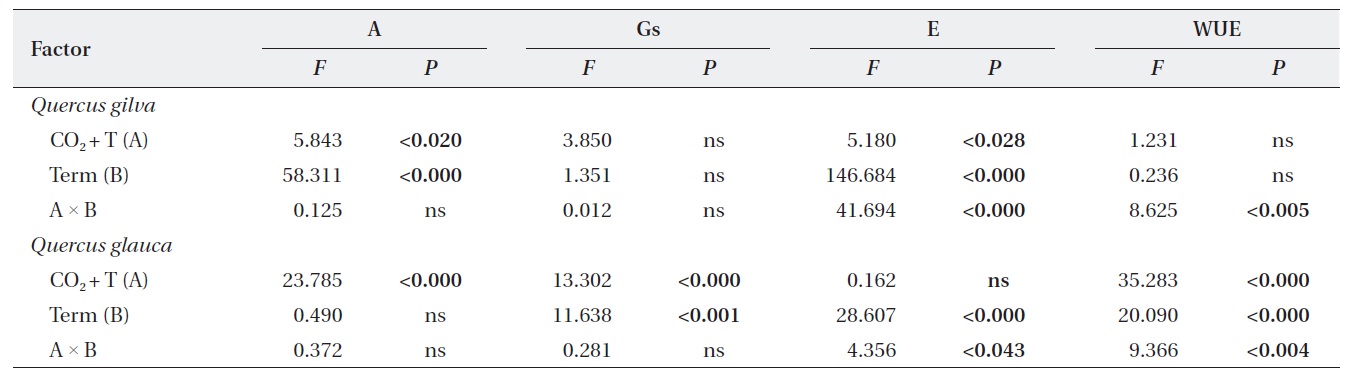
Effects of elevated CO2 T and term of CO2 exposure and their interactions on photosynthetic parameters of Quercus gilva and Q. glauca
The transpiration rate of both oak species was largely decreased after long-term exposure to elevated CO2 and temperature. WUE of
>
Effects of elevated CO2 and temperature on leaf C:N ratio, nitrogen and carbon contents
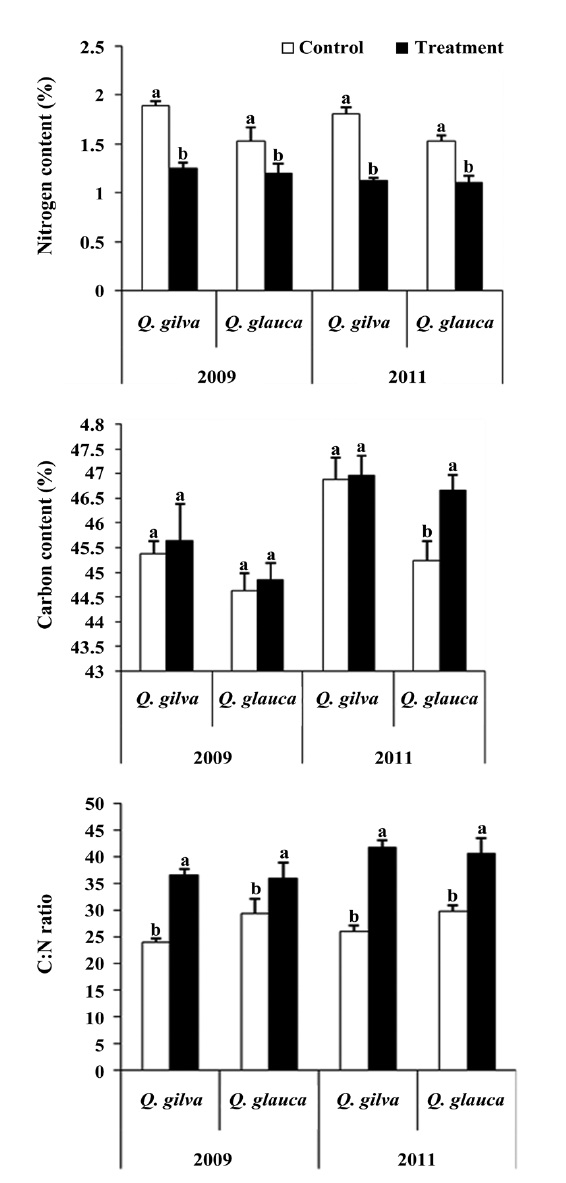
Elevated CO2 and temperature resulted in a decline in leaf nitrogen content for two oak species throughout this study (Table 3 and Fig. 2). The nitrogen content for
>
Effects of term of CO2 exposure on leaf C:N ratio, nitrogen and carbon contents
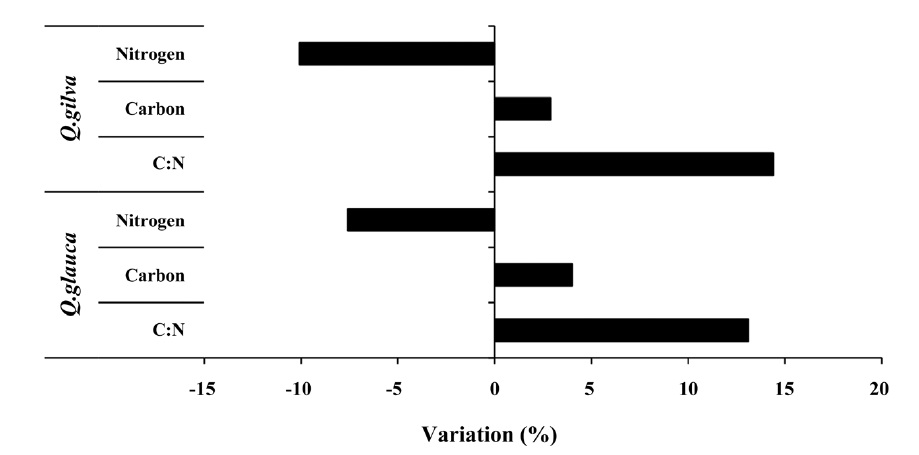
The pooled data for
14.4% (Fig. 3).
For
Down-regulation of photosynthesis was observed for
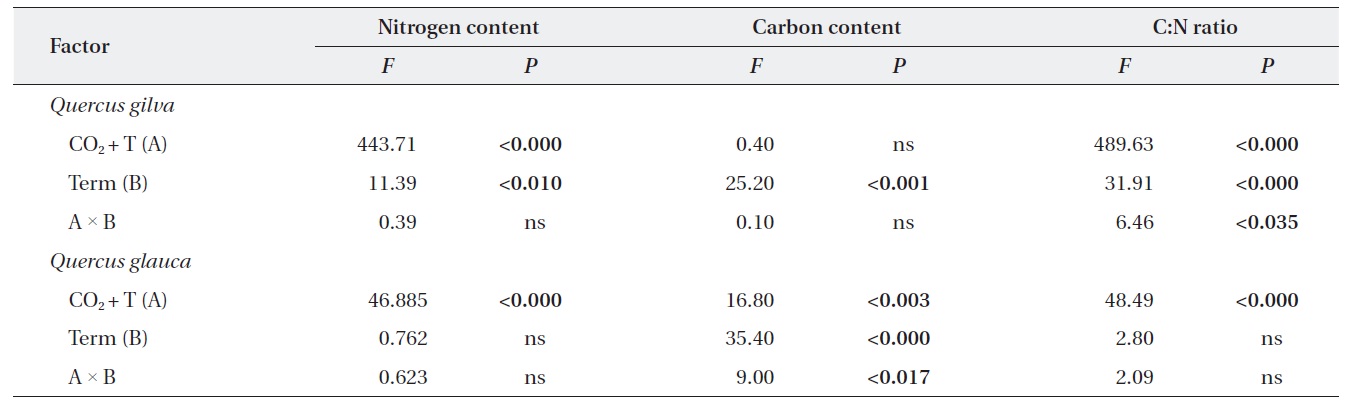
Effects of elevated CO2 T, term of CO2 exposure and their interactions on leaf nitrogen content, carbon content and C:N ratio for Quercus gilva and Q. glauca
grown at elevated CO2 concentration was 40% lower than for those grown at ambient CO2 concentrations. The CO2- induced enhancement of photosynthesis of
The down-regulation of photosynthesis of plants grown in elevated CO2 and temperature is often associated with starch accumulation (Tissue et al. 1993), reduction in rubisco content and activity (Albert et al. 2011), acclimation of photosynthetic capacity, such as the maximum carboxylation rate and the maximum rate of electron transport (Ainsworth and Rogers 2007), and, in particular, with leaf N content (Chaturvedi et al. 2009).
Many researchers have reported changes in the nitrogen contents of plant parts under such conditions (e.g., Cotrufo et al. 1998). The prolonged exposure to elevated CO2 concentration and temperature a leads to reduction in leaf nitrogen content associated with a down-regulation of photosynthesis (Kuehny et al. 1991). The strong relationship between net CO2 assimilation and leaf N based on mass is a principal functional relationship frequently utilized for predicting photosynthesis in many current models of plants at high CO2 levels (Liu et al. 2009) because the light capture, electron transport and the carbon metabolism portion of photosynthesis require large investments of nitrogen in the form proteins (Crous et al. 2010). According to Adam et al. (2004), the photosynthetic acclimation of
We identified significant effects of elevated CO2 + T and term of exposure on photosynthetic rates and leaf N contents of
The instantaneous photosynthetic rate of
Unlike
One of the most consistent effects of growth at elevated CO2 is a decrease in stomatal conductance (Ainsworth and Rogers 2007), leading to decreased transpiration rate and improved WUE (Leakey et al. 2009).
Significant elevated CO2 + T and term of exposure effects on stomatal conductance were detected in
The pooled data of
At the beginning of this study, elevated CO2 and temperature stimulated transpiration rates of both oak species, although, for
Higher temperatures increase transpiration and stomatal conductance by changing the vapor pressure deficit at the leaf surface. According to Valle et al. (1984), the transpiration of trees grown with high CO2 was similar that of ambient CO2 because the increases in leaf resistance caused by elevated CO2 were partially offset by increases in vapor pressure gradient between leaf surface and air, caused by increased transpiration. Also, stomatal conductance of the Yucca species was found to be increased under ambient CO2 when high temperature was applied (>45℃) whereas those grown under elevated CO2 did not increase stomatal conductance when exposed to high temperatures (Huxman et al. 1998), and furthermore, elevated CO2 largely offset the interactive effects of high temperature on water relations and photosynthesis in seedlings of
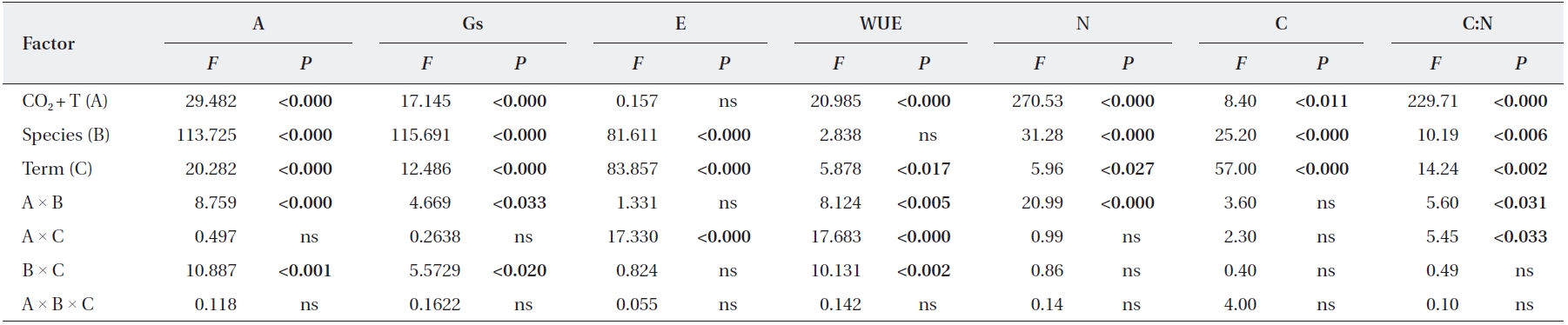
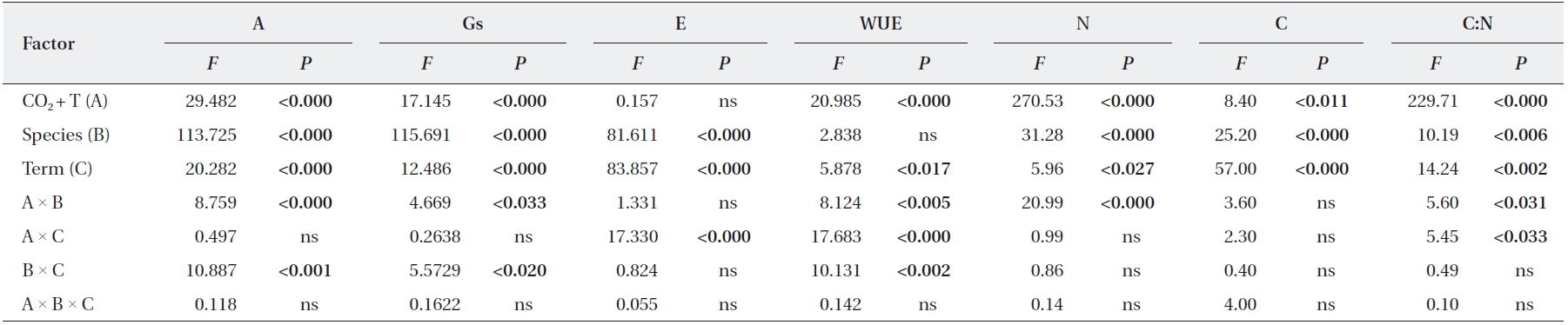
Statistical results from the repeated measures multivariate analysis of variance (MANOVA) evaluating the effects of species, CO2 T, term, interactions on photosynthesis and foliage biochemical characteristics measured for Quercus gilva and Q. glauca
Our results suggest that two species,
A well known response of plants to elevated CO2 and temperature is the production of leaves with a higher C:N ratio. Increased C:N ratio is caused by a relative reduction of nitrogen content and/or an increase in carbon content (Curtis 1996). In our study, the leaves of
Unlike
Plant responses to elevated CO2 and temperature are generally species-specific. We found that
After long-term of exposure to elevated CO2 and temperature, no change in the photosynthetic rate of
Unlike
Thus, it is concluded that the