Environmental pollution by heavy metals is a serious and complex problem that all the time occupies worldwide attention. Heavy metals are classified as the chief surface and groundwater pollutants. Industrial and municipal wastewater frequently contains metal ions that can be harmful to aquatic life and human health. Heavy metals are usually found in industrial wastewater; for example, water from textile production, paint manufacturing, leather tanning, etc. [1,2]. In general, heavy metals are not biodegradable and tend to accumulate in living organisms, causing various diseases and disorders. In adults, Pb(II) can increase blood pressure and cause fertility problems, nerve disorders, muscle and joint pain, irritability, and memory or concentration problems [3]. The treatment methods for metal-bearing effluents commonly include chemical precipitation, membrane filtration, electrolytic reduction, solvent extraction, ion exchange, and adsorption [4]. Among these treatments, adsorption is considered to be an effective and economic method that has attracted considerable interest. Activated carbons are adsorbents that are used industrially in multiple processes for product separation and purification, and for the treatment of liquid and gaseous effluents. Considering, however, the high cost of activated carbon and the tedious procedures for preparation and regeneration of activated carbon, there is a continuing search for low-cost potential adsorbents. Lignocellulosic materials and wastes such as peanut skin, cotton, onion skin, rice hulls, maize stalks, bark, jute fibers, bagasse, rice straw, corncobs, and palm kernel husks have received much attention in the area of heavy metal ion removal [5]. Various functional groups such as carboxylates, phenolic and aliphatic hydroxyls, and carbonyl groups in these materials have the ability to adsorb some metal ions. In order to increase the metal ion adsorption in cellulose, it was chemically modified by introducing different groups such as phosphoric acid (H3PO4) or potassium carbonate (K2CO3) [6].
2.1.1. Physical activation
The walnut shells were obtained from local natural resources. After obtaining them, the fresh walnut shells were washed several times with distilled water to remove surface impurities; shells were then dried at room temperature for one day. The samples were heated and burned and the charcoal was crushed with a grinder and then ground to pass through a 100-mesh sieve for further experiments. It was activated to a final temperature of 500℃ in a muffle furnace for 6 h.
2.1.2. Chemical activation
Chemical activation methods using H3PO4 or K2CO3 were used to activate the raw material. One gram of each raw material was weighed and then the weighed raw sample was impregnated in 5 mL 50% (v/v) concentration of phosphoric acid and 5 mL 20% (w/v) concentration of potassium carbonate for 1 h at room temperature. After that, the blend was transferred to a muffle furnace, where carbonization was carried out under an air atmosphere. The plant material was chemically modified with H3PO4 and K2CO3 in three steps of temperatures and times. The furnace was heated at 200℃, and maintained at this temperature for 30 min in order to allow the free evolution of water; eventually, a black sticky solid was obtained. Then, the furnace was heated at 500℃ , and maintained at this temperature for 60 min. Finally, the furnace was heated at 700℃ , and maintained at this temperature for 15 min. After cooling to room temperature, the solid was washed with ultra pure water at 25℃ to remove excess H3PO4 or K2CO3. The carbon samples were dried at 50℃ < t <80℃.
2.2. Batch equilibrium studies
Activated carbon (0.05 g) was added to solutions of Pb(NO3)2 with different initial concentrations. The mixture was stirred for 15 min at room temperature (25℃ ). The amount of remaining metal ions was determined by an atomic absorption spectrophotometer (Shimadzu, Japan). The amount of Pb(II) adsorbed on the adsorbent at adsorption equilibrium was calculated according to the following Eq. (1)
where C0 and Ce (mg/L) are the initial and equilibrium Pb(II) concentrations, respectively. V is the volume of the solution (L), and W is the mass of adsorbent used (g).
In the order to study the effect of contact time between adsorbent and adsorbate, at first, we put 20 ppm of Pb(II) and 0.05 g of activated carbon in contact for 240 min with 0.05 g of activated carbon. The maximum adsorbtion took place in the first 15 min of contact.
To analyze the effect of initial Pb(II) concentration, at first we changed the concentration of Pb(II), in a range from 5 to 23 ppm. Then, by putting the samples in contact with the adsorbent for 15 min, at this stage, the maximum absorbtion of 20 ppm concentration of Pb(II) took place.
The effect of the pH on the metal adsorption by modified activated carbon was studied for pH 3, 4, 5, 6, 7, 8, 9, and 11, all points at which the material exhibits chemical stability.
The effect of activated carbon from walnut shells (ACW) dosage on the adsorption process was investigated by varying the sorbent dosage from 0.01-1 g of Pb(II) solution. The experiment was conducted by measuring 20 ppm of Pb(II) solution. Appropriate dosage of the ACW was measured into solution and it was agitated for 15-240 min.
According to the Langmuir model, adsorption occurs uniformly on the active sites of the adsorbent, and once an adsorbate occupies a site, no further adsorption can take place at this site. Thus, the Langmuir model is given by Equation [7]
where qmax and b, the Langmuir constants, are the saturated monolayer adsorption capacity and the adsorption equilibrium constant, respectively. A plot of Ce/qe versus Ce would result in a straight line with a slope of (1/qmax) and intercept of 1/ bqmax The Langmuir parameters can be used to predict the affinity between the sorbate and adsorbent using the dimensionless separation factor RL:
The RL value indicates the shape of the isotherm as follows [8]:
RL > 1 Unfavorable
RL =1 Linear
0 < RL < 1 Favorable
RL = 0 Irreversible
The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution. The empirical model has been shown to be consistent with the exponential distribution of the active centers, a characteristic of heterogeneous surfaces. The amount of Pb(II) adsorbed onto the modified ACW at equilibrium, qe, is related to the concentration of Pb(II) in the solution, Ce, as follows [9]:
This expression can be linearized to give
where Kf and n are the Freundlich constants, which represent adsorption capacity and adsorption intensity, respectively. A plot of lnqe versus lnCe would result in a straight line with a slope of (1/n) and intercept of lnKf.
The Dubinin-Radushkevich (D-R) isotherm was employed in the following linear form [10].
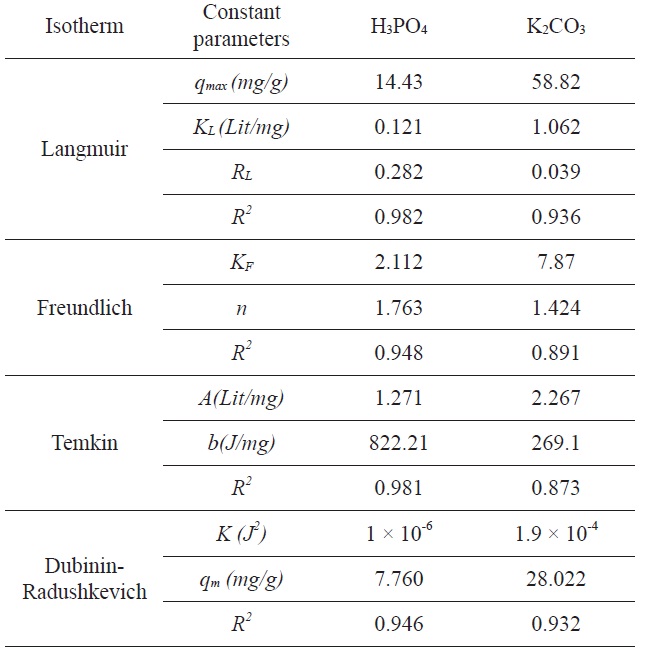
Comparison of the coefficients of isotherm parameters for adsorption of Pb(II) on activated carbon modified by H3PO4 or K2CO3
The Polanyi potential ε can be expressed as
The Temkin equation suggests a linear decrease of sorption energy as the degree of completion of the sorptional centers of an adsorbent is increased. The Temkin isotherm has been generally applied in the flowing form [11,12].
where B=RT/b, R is the gas constant, b is the Temkin isotherm constant, T is the absolute temperature (K), and A is the Temkin isotherm constant (L/mg). Therefore, by plotting qe versus ln Ce one can determine the constants A and B.
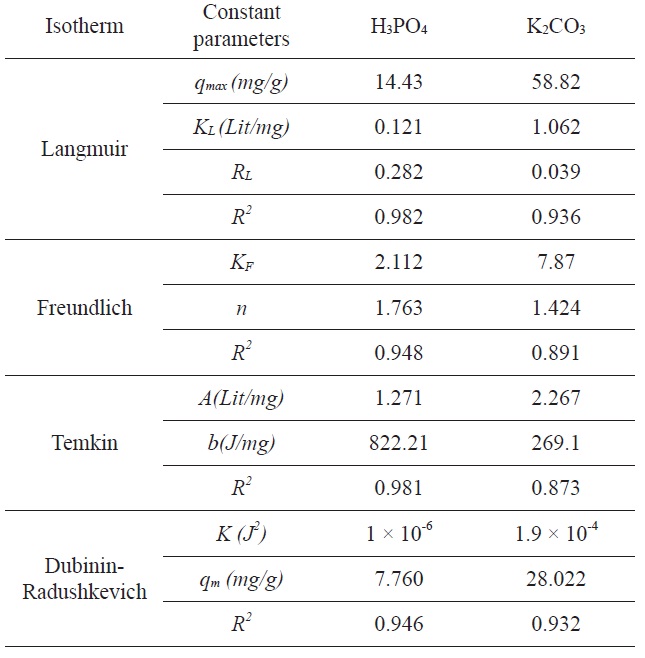
A list of the parameters obtained, together with the R2 values, is given in Table 1. The experimental results were analyzed by using Langmuir, Freundlich, Tempkin, and D-R isotherm models. The correlation coefficients for Langmuir and Tempkin equations fit better than those of the Freundlich and D-R equations for activated carbon modified by H3PO4 and the correlation coefficients for the Langmuir and the D-R equations fit better than did the Freundlich and Tempkin equations for activated carbon modified by K2CO3. The experimental data indicated that the adsorption isotherms are well described by the Langmuir isotherm equation and the calculated adsorption capacity of activated carbon was 14.43 (mg/g) at 25℃ for activated carbon
modified by H3PO4 and 58.82 (mg/g) for activated carbon modified by K2CO3. Based on Table 1, it is clear that the correlation coefficient R2L is comparatively higher than R2F, R2T, and R2(D-R). The value of RL for the adsorption of Pb(II) with activated carbon by modified H3PO4 was 0.282, and the value of RL for the adsorption of Pb(II) with activated carbon by modified K2CO3 was 0.039. This value indicates that the adsorption behavior of activated carbon was favorable.
In order to elucidate the mechanisms and the possible rates of solute adsorption of solutions, different kinetic models have been applied.
The pseudo first-order equation was presented by Kavitha and Namasivayam [13] for sorption of oxalic acid and malonic acid onto charcoal. Afterwards, many researchers reported first order Lagergern kinetics for adsorption of different pollutants on different sorbents [14]. The linearized form of the pseudo first-order equation of Lagergren is generally expressed as follows [15]:
where qt is the amount of Pb(II) adsorbed (mg/g) at time t, k1 is the equilibrium rate constant of pseudo-first order kinetics (min-1) and t is the constant time (min). According to Eq. (9) the plots of
The linearized form of the pseudo- second order chemisorptions kinetic rate equation is presented below [16-19]:
where k2 (g/mg min) is the rate constant for pseudo-second order adsorption. The intercept and slope of
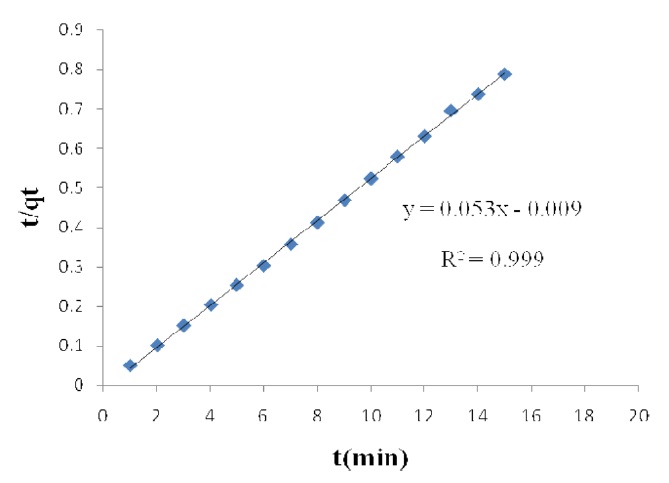
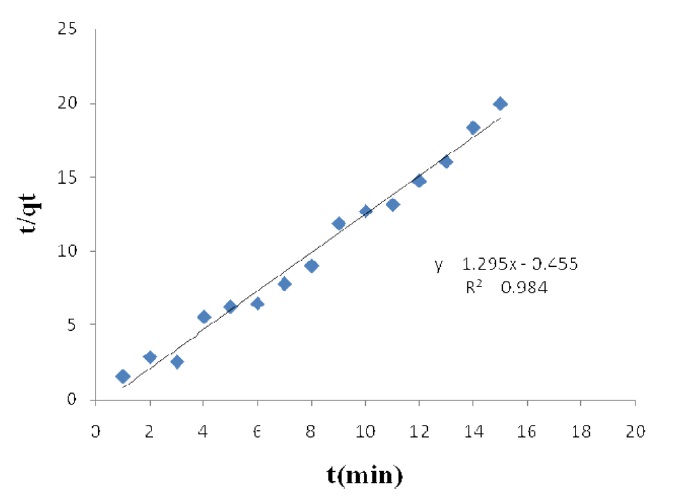
vs. t (Figs. 1 and 2) were used to calculate the pseudo-second order rate constants k2 and qe, respectively.
The results obtained from these studies were analyzed by using pseudo first order and pseudo second order kinetic models.
The calculated qe values agree very well with the experimental data; the correlation coefficients for the second order kinetic model are higher than 0.99 in all cases for activated carbon modified by K2CO3 and H3PO4. These data indicate that the adsorption of acid dyes from wastewater onto activated carbon obeys the pseudo second order kinetic model.
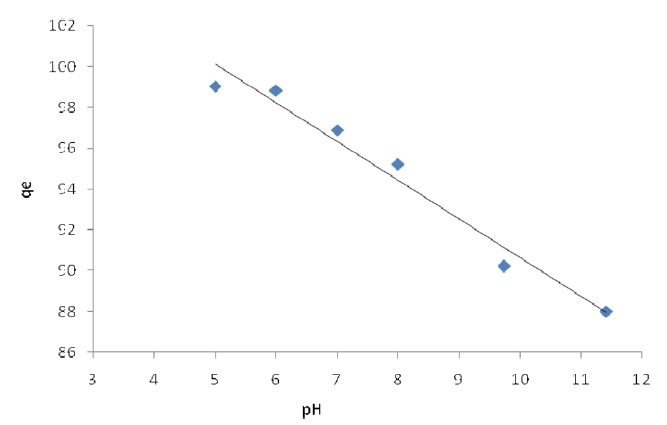
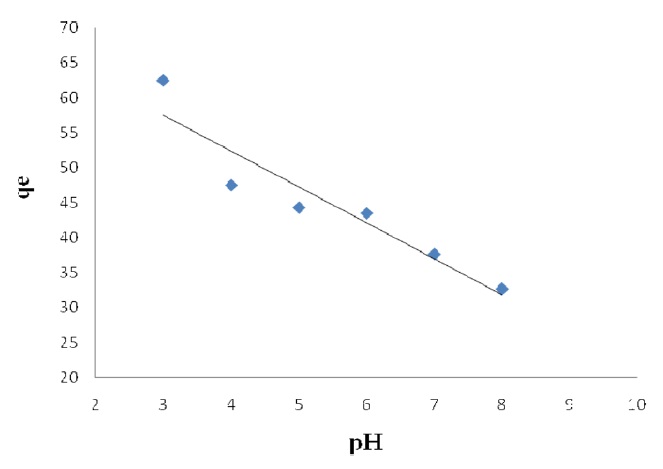
The pH level of the aqueous solution is an important variable for the adsorption of metals on the adsorbent. The effect of the pH on Pb(II) adsorption by modified activated carbon was studied for pH 3, 4, 5, 6, 7, 8, 9 and 11, at which levels the material exhibits chemical stability. The efficiency of Pb(II) sorption depends on the pH level of aqueous solutions. The influences of pH on the adsorption of Pb(II) onto modified activated carbon are shown in Figs. 3 and 4. It is apparent that using the solution at pH > 1 gives the highest removal of Pb(II) on the activated carbon modified by K2CO3. The data reveal that the adsorption capacity is low at pH<1 and that Pb (II) adsorption decreases with the pH decreases from 4 to 8 Pb(II) on activated carbon modified by K2CO3. The amount of adsorbed Pb(II) is the minimum rate at pH > 6 on activated
carbon modified by H3PO4; the maximum adsorption capacities were calculated at pH = 5.
The effect of contact time on the amount of Pb(II) adsorbed per gram of ACW by H3PO4 and K2CO3 was investigated. Equilibrium is reached after 15 min of immersion and it is a relatively fast adsorption process.
The reasons for the maximum adsorption mentioned above are the following:
1) Reduction of weight of activated carbon
2) Reduction in speed of pore development
3) Filling of pores during the activation time
3.5. Effect of the initial Pb(II) concentration
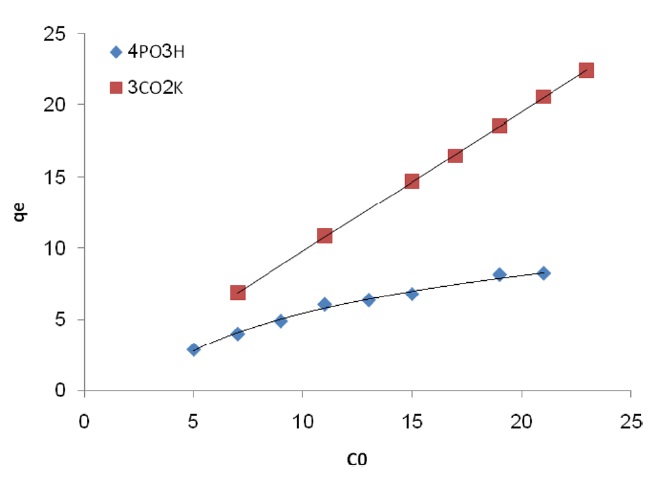
The effect of initial dye concentration has been studied and is presented in Fig. 5. The figure shows the effect of the Pb(II) concentration on the adsorption of the different types of Pb(II) at pH 5. The figure shows that the amount of Pb(II) adsorbed increases with the increasing concentration and then tends to level off. The maximum milligrams per gram of Pb(II) adsorption is 8.23.
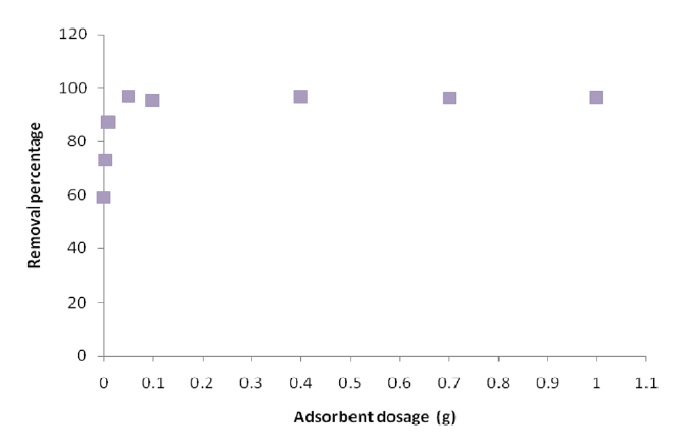
Fig. 6 shows the effect of ACW by H3PO4 and K2CO3 dose on the removal percentage of Pb(II). The effect of ACW dosage on the adsorption process was investigated by varying the sorbent dosage from 0.01-1 g of Pb(II) solution. The experiment was conducted by measuring 20 ppm of Pb(II) solution. The appropriate dosage of ACW was measured into the solution and solution was agitated for 15-240 min. Samples were withdrawn at fixed intervals and analyzed for residual Pb(II); the amount of sorbate sorbed per unit mass of the ACW was calculated by using the mass balance procedure.
Activated carbon adsorption of metallic ions (mainly Pb(II)), similar to or even better than that of commercially available products, was produced by the chemical activation of walnut shell.
The results showed that as the amount of the adsorbent increased, the percentage of Pb(II) removal increased accordingly.
The optimum pH value for lead adsorption was determined to be 5.The maximum removal of Pb(II) was obtained at pH 5, with a removal level of 98.84% for activated carbon modified by H3PO4 and 99.03% for activated carbon modified by K2CO3 and an adsorbent dose of 0.05 g and 20 mg/L initial Pb(II) concentration at room temperature.
The experimental results were analyzed using the Langmuir, Freundlich, Tempkin, and D-R isotherm models. The correlation coefficients for the Langmuir and Tempkin equations fit better than did the Freundlich and D-R equations for activated carbon modified by H3PO4; the correlation coefficients for the Langmuir and D-R equations fit better than did the Freundlich and Tempkin equations for activated carbon modified by K2CO3.
The results of this investigation show that activated carbon modified by H3PO4 and K2CO3 has a suitable adsorption capacity for the removal of Pb(II) from aqueous solutions.