Ammonia (NH3) is a kind of gas with high toxicity. The detection of NH3 is necessary and important for environmental monitoring and chemical control in industrial, agricultural, and medical fields. For example, the maximum NH3 concentration allowed at the industrial working place is 25 ppm for 8 h exposure, and 35 ppm over a 10 min period [1]. However, the olfactory limit of detection of NH3 is 55 ppm [2]. Therefore, a sensitive NH3 gas sensor must be developed for the detection of lower NH3 concentration below these limits.
NH3 gas sensors based on conventional materials such as SnO2 [3], TiO2 [4], In2O3 [5], WO3 [6], and ZnO [7] have been developed with good responsiveness and selectivity, and fast response-recovery, that can be used in detecting the lower level presence of NH3 gas. However, these NH3 gas sensors are fabricated using metal oxides which operate effectively at temperature ranges between 150 and 400℃, resulting in both high power consumption and complexities in integration. Thus, there is a great need to develop a new class of materials for gas sensors that have good performance at room temperature.
Carbon nanotubes' (CNTs) special geometry and their outstanding feature of surface reactivity, offer great potential for application in gas sensor devices working at room temperature [8]. However, CNTs still have certain limitations for gas sensor application, such as long recovery time, limited gas detection, and vulnerability to humidity and other gases. These limitations can be resolved effectively by forming composites with conducting polymers.
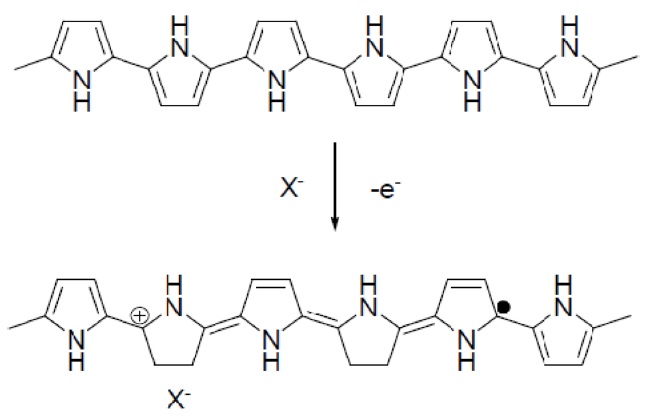
Conducting polymers such as polypyrrole (PPy) and polythiophene and their composites have been widely investigated as effective materials for chemical sensors [9-11]. Among the conducting polymers, PPy has attracted considerable attention because it varies its electrical conductivity using the redox reactions and has a good stability in both air and aqueous media [12]. Fig. 1 demonstrates the oxidation process of PPy. Some electrons are removed from PPy backbones by chemical or electrochemical oxidations, leaving positive charges on them. The resulting cation radicals are called polarons, acting as the charge carriers. Counter ions, X-, are also induced close to the polymer chains to balance the positive charges. Doped conducting
polymers are semiconductors or conductors (100-105 S cm-1). PPy and other conductive polymers have, therefore, also been classified as organic metals. There exists a wide range of applications suitable for organic metals use, such as cell culture substrates [13], field effect transistors [14], light-emitting diodes [15], solar cells [16,17], electrochromic devices [18], electronic circuits [19], elastic textile composites [20], supercapacitors for energy storage and secondary batteries [21], protection of metals [22], ion exchange membranes responding to external stimulations [23], and sensors and biosensors [24,25].
Preparation of PPy/multi-walled CNTs (MWCNTs) composites by electropolymerization on an oxidisable surface such as a Cu electrode have been reported, using MWCNTs as an appropriate substrate to induce the preferential deposition of PPy [26]. In our study, PPy/MWCNTs composites were prepared conveniently by in situ chemical oxidative polymerization resulting in the uniform deposition of PPy on MWCNTs without using any electrode.
Gas sensors were fabricated by spin-coating the PPy/MWCNTs composites having various compositions. The NH3 sensing properties of PPy/MWCNTs composites were investigated based on the variation of electrical resistance upon gas adsorption. This study aims to investigate the synergistic effects in improving the gas sensing properties based on the combination of electrical properties of MWCNTs and PPy.
Pyrrole monomer (99%), ammonium persulfate (APS), and MWCNTs were obtained from Sigma Aldrich. The diameter of the MWCNTs was between 110 and 170 nm, and purity of the MWCNTs was over 90%. Sodium dodecyl sulfate as surfactant was obtained from ICN Biomedicals. Hydrogen peroxide (H2O2) was purchased from Kanto chemical.
2.2. Synthesis of PPy and PPy/MWCNTs composites
PPy was synthesized by free radical chemical oxidative polymerization through the direct route using H2O2 as oxidant. Three gram of pyrrole monomer was dropped into distilled water and stirred continuously for 10 min. Then 4.5 mL of H2O2 was added to the pyrrole solution. 0.8 g of APS dissolved in 10 mL distilled water was slowly added to the pyrrole solution and the reactant solution was polymerized for 4 h at 0℃ with constant stirring. The synthesized PPy was filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The PPy powder was dried under vacuum at 40℃ for 24 h.
PPy/MWCNTs composites were synthesized using in situ chemical oxidative polymerization on the MWCNT template. The polymerization of pyrrole was carried out in distilled water using H2O2 as oxidant for pyrrole and APS as initiator. Various contents of MWCNTs (0, 0.5, 1.0, 3.0, and 5.0 wt%) were dispersed in the surfactant solution and ultrasonicated over 1 h. Three gram of pyrrole monomer was added into the MWCNTs dispersed mixture and stirred continuously for 10 min. Then 4.5 mL of H2O2 was added to the reactant solution followed by the slow addition of 0.8 g of APS, dissolved in 10 mL distilled water, to the reactant solution. The template polymerization was carried out for 4 h at 0℃ with constant stirring. The synthesized PPy/MWCNTs composites were filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The composite powders were dried under vacuum at 40℃ for 24 h. Five different composite samples, such as PPy/0 wt%-MWCNTs, PPy/0.5 wt%-MWCNTs, PPy/1 wt%-MWCNTs, PPy/3 wt%-MWCNTs, and PPy/5 wt%-MWCNTs, were named as PPy, 0.5-PPC, 1-PPC, 3-PPC, and 5-PPC, respectively.
2.3. Preparation of the gas sensor
The PPy/MWCNTs composites (0.1 g) were dispersed in acetone (5 g) and ultrasonicated for 30 min to disperse the samples uniformly in the acetone. Then the dispersion (0.01 g) was dropped onto a silicon wafer by using a micro-pipette (Ovation pipette, Vistalab) and spin-coated (spin coater, ACE-200) at 900 rpm for 4 min. The coated wafer was heated to remove the solvent at 40℃ for 10 min. The thickness of the coating layer was 15 ± 1 μm .
2.4. Measurement of gas sensing properties
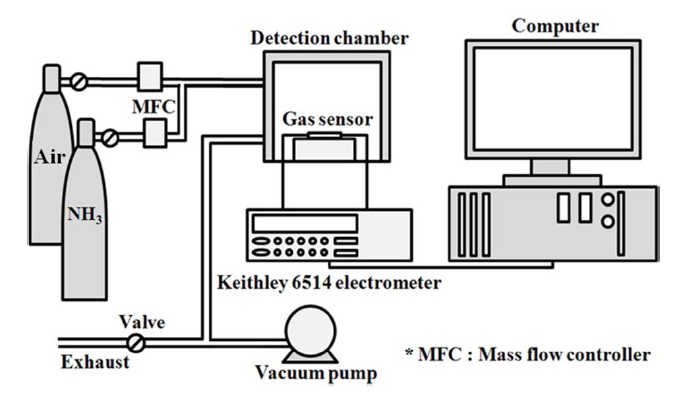
In order to evaluate the gas sensing properties of composites, their electrical resistance was measured using a programmable electrometer (Keithley 6514). The setup of the gas sensor system is depicted in Fig. 2. The gas sensing device was prepared by using two Pt electrodes and a SiO2 plate, as shown in previous work [27,28]. This measurement was performed in a stainless steel chamber with a volume of 1500 cm3. The chamber was connected to gas cylinders (NH3 and air). The prepared sample gas sensor was placed in a sealed chamber under vacuum at a pressure of 1 × 10?3 torr. Air was injected into the chamber to stabilize the electrical resistance of the gas sensor. The air was used as the carrier gas for the control of gas concentrations. The mixture of two different gases was then prepared with a concentration of 33.2 ppm NH3 in air and injected into the chamber. The gas feeding rate was kept constant at 500 sccm in all cases. The change in electrical resistance was measured at 298 ± 1 K. When the electrical resistance was stable under the NH3 gas in-
jection, the injection of NH3 gas was turned off and the recovery of electrical resistance was observed. The adsorbed gases on the gas sensors were removed by heating the sensors to 373 K at a pressure of 1 × 10-3 torr for 5 min [27,28]. Recovery testing was carried out four times.
The gas sensitivity (S) is expressed by the following equation [29,30]:
where R0 is the resistance due to the air and Rg is the resistance measured upon exposure to NH3 gas.
Field emission scanning electron microscopy (FE-SEM, Hitachi, S-5500) was used to investigate the surface morphology of the prepared gas sensors. FE-SEM measurements were conducted at 5 kV. SEM images were taken without prior treatment
in order to ensure the acquisition of accurate images. Thermogravimetric analysis (TGA) was performed under nitrogen flow (50 cm3 min-1) at a heating rate of 10℃ min-1 to 800℃ by using Perkin Elmer TGA5OH thermogravimetric analyzer.
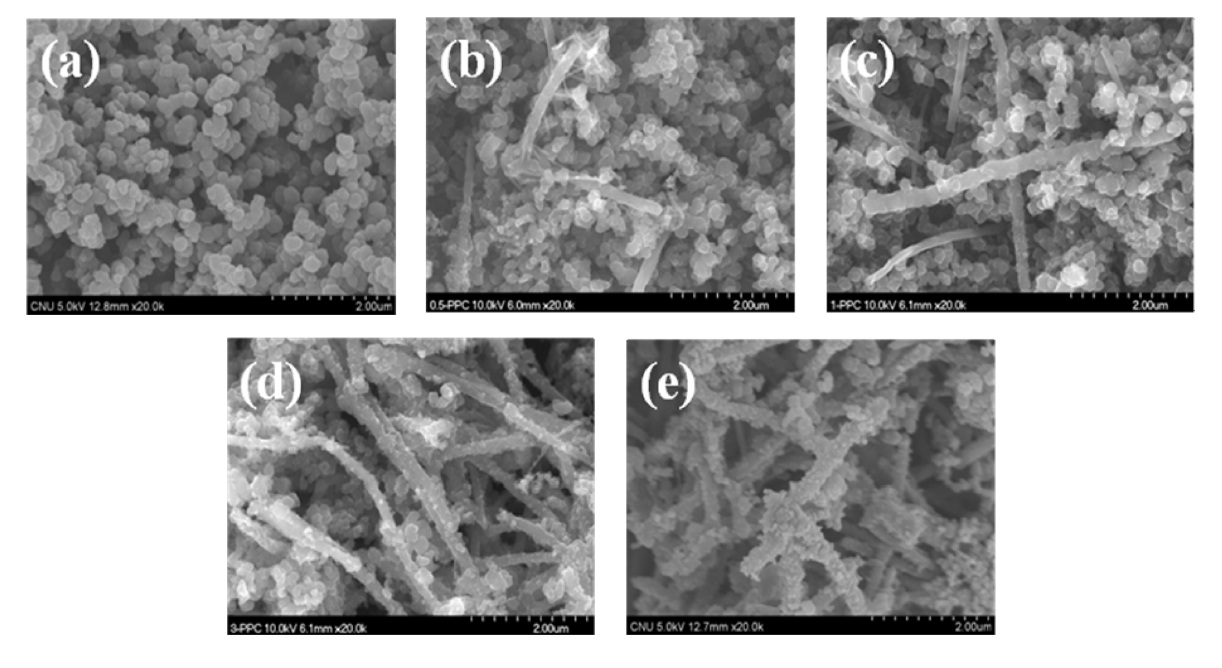
FE-SEM images of PPy and PPy/MWCNTs composites (0.5- PPC, 1-PPC, 3-PPC, 5-PPC) are presented in Fig. 3. The granular structure of PPy is seen in Fig. 3a. PPy was coated uniformly on the MWCNTs templates while maintaining the granular structure as shown in Figs. 3b-e.
The layer structures of PPy were formed successfully on the outer surface of MWCNTs. In addition, the size reduction of PPy clusters was observed as MWCNTs content increased. These morphological characteristics were expected to play an important role in the NH3 sensing performance of the PPy/MWCNTs composites.
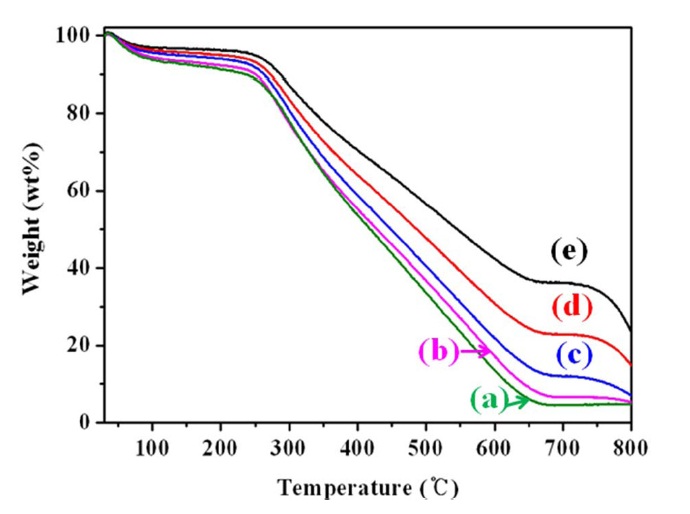
The thermal stabilities of PPy and PPy/MWCNTs composites were investigated by TGA as shown in Fig. 4. The PPy/MWCNTs composites showed improved thermal stability compared with the PPy alone. MWCNTs are well known scavengers of free radicals [31]. MWCNTs, which were dispersed uniformly in the PPy matrices, retarded the thermal decomposition of the composites effectively. Although the PPy showed poor thermal stability, with an initial decomposition temperature of 250℃ [32], the thermal properties of the PPy/MWCNTs composites were improved by incorporating the MWCNTs.
3.3. Gas sensitivity by resistive response
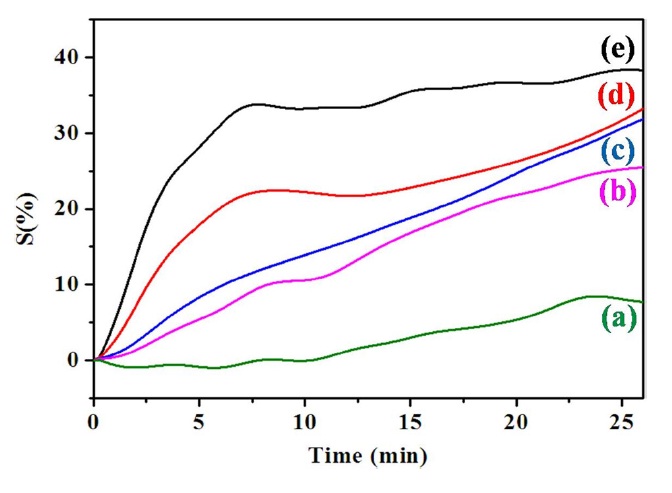
Fig. 5 presents the NH3 gas sensitivity of various PPy/MWCNTs composites as measured by changes in the electrical resistance. The gas sensitivity of PPy and various PPy/MWCNTs composites was investigated based on two different factors, namely, the response time and the sensing intensity. PPy/ MWCNTs composites usually exhibited a change in electrical resistance in accordance with the typical characteristics of a ptype semiconductor. When this occurs with a p-type conducting polymer, the doping level as well as the electric conductance of the conducting polymer is enhanced. Generally, the electrons travel from CNTs to the NH3 gas (reducing gas), resulting in the increase of electrical resistance [33]. The following reactions are possibly involved in the NH3 gas sensing process [34,35]:
PPy+ + NH3 → PPy0 + NH3+ Adsorption
PPy0 + NH3+ → PPy+ + NH3 Desorption
Based on this mechanism, all p-type conducting polymers are
expected to de-dope under an NH3 gas atmosphere. It was found that a resistance increase occurred upon reaction with NH3 gas.
In this study, the gas sensitivity of various PPy/MWCNTs composites was evaluated to investigate the synergistic effects of PPy and MWCNTs. The variation in electrical resistance is attributed to the electron charge transfer between NH3 gas and the surface of PPy/MWCNTs composites. The PPy sample showed continuously increased resistivity during the initial 25 min, with the slow response to NH3 gas showing the lack of sensitivity in gas sensing properties. This drawback was overcome by forming a composite with MWCNTs.
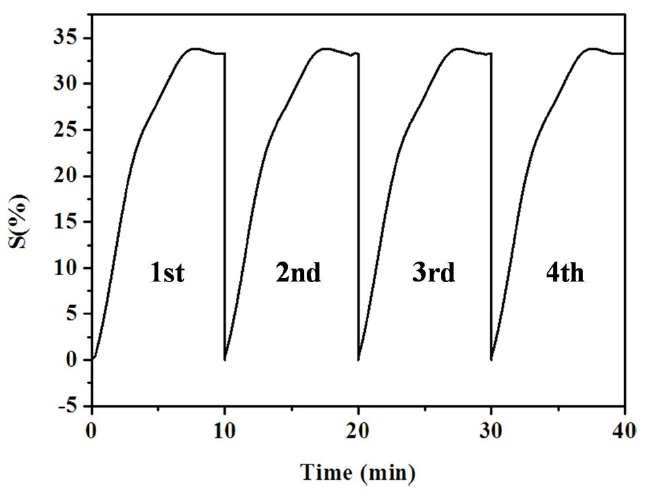
The sensitivity for NH3 gas was improved significantly as MWCNTs content was increased in the PPy/MWCNTs composites. The 5-PPC sample showed not only the highest sensitivity change of 65% but also the fastest response time of less than 10 min for NH3 gas among the prepared PPy/MWCNTs composite samples. 5-PPC showed about 15 times higher sensitivity for NH3 gas compared with PPy due to the synergistic effect of PPy and MWCNTs based on the combination of electrical properties of the MWCNTs and PPy. The conducting polymer-coated MWCNTs showed the unique combination of effects on both the efficiency of adsorption of gas and the resistive response, resulting in the improvement in gas sensing properties. The improved sensitivity for NH3 gas seems to be attributable to the effective electron charge transfer in the interface between PPy/MWCNTs composites and NH3 gas, and the resulting change in electrical resistance by the uniformly dispersed MWCNTs. The reproducibility of the gas sensor performance was also investigated every 10 min by evacuating the gas in the chamber at a pressure of 1× 10-3 torr. The gas sensing behavior of the 5-PPC in the cyclic exposures to NH3 gas is shown in Fig. 6. Excellent reproducibility was observed for the 5-PPC sample, showing over 90% recovery.
The composites of PPy and MWCNTs were prepared by in situ chemical oxidative template polymerization on the surface of MWCNTs, as effective NH3 gas sensing materials. PPy was formed uniformly on the surface of the MWCNTs as confirmed by FE-SEM images. The grain size of the PPy decreased as MWCNTs content increased. The thermal stability of the PPy coating on MWCNTs was improved by the effect of oxygen radical scavenger of the MWCNTs. The improved gas sensitivity for NH3 was attributed to the effective electron charge transfer at the interface between PPy/MWCNTs composites and NH3 gas, and the efficient electron transfer by MWCNTs. PPy/MWCNTs composites showed the synergistic combination effects of efficient adsorption and conduction resulting in the improvement of gas sensing properties by dispersing MWCNTs uniformly in PPy. The reproducibility of gas sensing behavior was also investigated every 10 min by evacuating the gas in the chamber at a pressure of 1 × 10-3 torr. Excellent reproducibility was observed for the PPy/MWCNTs composites showing over 90% recovery. The high-performance NH3 gas sensor could be prepared based on the composites of PPy and MWCNTs.