Carbon nanotubes (CNTs) are one-dimensional nanomaterials that are considered as ideal reinforcing agents for polymer matrices because of their unique structure and properties [1,2]. Electrically conductive composites filled with CNTs have attracted increasing attention for a variety of applications, such as static-charge dissipation [3], electromagnetic interference shielding [4], and actuators [5]. However, CNTs are often in bundles or they are entangled because of very strong intertubular van der Waals attractions, which is the current bottleneck in their application [6].
Chitosan (CS) is a linear polysaccharide synthesized by the deacetylation of chitin, a natural polymer found in the exoskeleton of crustaceans. CS is widely used in biomedical applications, drug delivery, food industry, biotechnology, pharmaceuticals, biomedicine, packaging, wastewater treatment, cosmetics, etc. [7,8]. Another advantage of CS is its solubility in acidic aqueous media. Natural polymers modified with suitable nanofillers have now found potential applications as electrochemical sensors and electrodes [9-13]. CS can be made to possess amphiphilic properties giving it a unique capacity to solubilize hydrophobic CNTs in aqueous solution [14,15]. A key characteristic of the CNT/CS composite is its conductivity, as defined by the charge transfer from one conductive particle to another. Because conduction of electrical charge is established when a network of conductive CNTs reaches a critical percolation threshold density that provides direct electrical contact between particles, the effective conductivity of a CNT/CS composite depends upon many factors, such as size, shape, density, and distribution of CNTs within the CS matrix, as well as chemical interactions between the two materials [16-18].
A Fe3O4/CNT/CS composite is expected to have diverse properties because each component contributes different chemical and physical properties to the composite. A Fe3O4/CNT/CS composite may find applications in drug delivery, tumor treatment, enzyme en-
gineering, batteries, electro-magneto rheological fluids, electromagnetic shielding and magnetic recording. In this study, Fe3O4/ CNT/CS nanocomposite films were prepared by the solution casting method. The main objective was to investigate the synergistic effect of Fe3O4 and CNTs on the electrical properties of the nanocomposites. The films were prepared with different concentrations of Fe3O4 at fixed quantities of CNTs in order to determine the optimal metal loading for improving conductivity. Subsequently, the electrical conductivity and X-ray diffraction (XRD) patterns were determined for the nanocomposite films.
CS (average molecular weight = 350 000 gmol-1, 90% degree of deacetylation was purchased from Sigma Aldrich. Raw multiwalled CNTs (MWCNTs, CM-95), synthesized using the chemical vapor deposition method, were purchased from Hanhwa Nanotech Co. Ltd., Korea. The MWCNTs had diameters of 10-15 nm, tube length of 10-20 μm and a purity of 95%. Magnetite (Fe3O4) nanopowder, (<50 nm particle size [transimission electron microscopy], ≥98% trace metals basis) was purchased from Sigma Aldrich. Acetic acid was used to dissolve CS in distilled water.
CS nanocomposite films containing Fe3O4 and CNTs were prepared by the solution casting method [19]. The concentrations of the functional additives (Fe3O4 and CNT) were changed in order to evaluate the synergistic effect of Fe3O4 and CNTs in the nanocomposite films. Electrical conductivities of the films were measured at room temperature using a ring probe method with a high resistivity meter (MCP-HT 450, Mitsubishi). Wide angle XRD patterns of the Fe3O4/CNT/CS nanocomposite films were recorded with a Rigaku Rotaflex (RU-200B) X-ray diffractometer using Cu Kα radiation with a Ni filter. The tube current and voltage were 300 mA and 40 kV, respectively, and 2θ angular regions between 0 and 40° were explored.
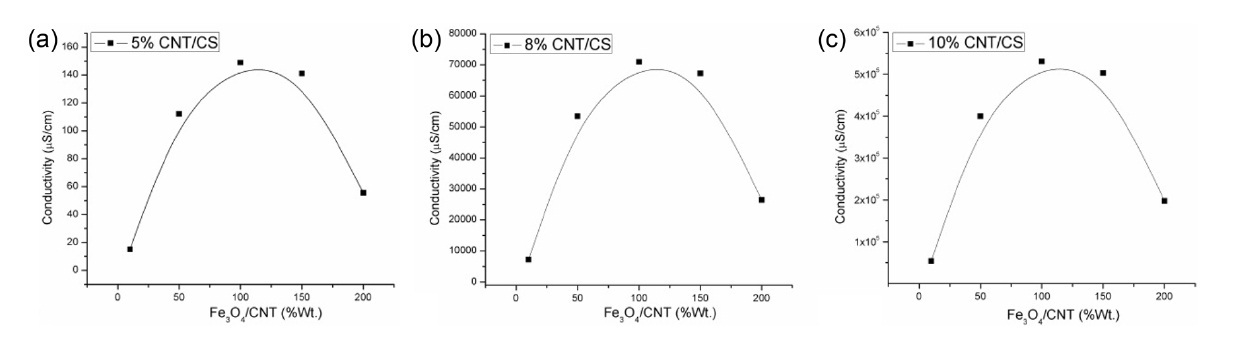
The CS composites were characterized in relation to their conductivity as a function of the CNT to Fe3O4 ratio. This was important because the establishment of a highly conductive CNT/CS film requires a network of effective tube-tube contacts. The quality of such a network is ultimately defined by the nanotube concentration and the relative extent of homogeneous (i.e.,
well-distributed within the matrix) to heterogeneous distribution (i.e., formation of aggregates). The nanotube dimensions limit the effectiveness of electron tunneling across tube-tube contacts. It was also expected that Fe3O4 addition would be beneficial to the electrical conductivity of the CNT and the subsequent composite because of the inherent electrical conductivity of Fe3O4. Furthermore, the nanoparticles could facilitate electron transfer between nanotubes while being dispersed in the polymer matrix because the composite would acquire more conductive channels and subsequently, a higher metallic character.
Fig. 1a shows how the effect of Fe3O4 loading, expressed as a weight percentage relative to the CNT content, affects the conductivity of the nanocomposite film. The results clearly indicate the dependence of conductivity on the Fe3O4 to CNT ratio. The conductivity improved with increasing Fe3O4 content, reaching a maximum at 100% loading, with a subsequent decrease with higher Fe3O4 content. It is clearly established that a 1:1 ratio of Fe3O4 to CNT in the CS nanocomposite film is the optimal loading for conductivity enhancement. This behavior in conductivity was observed at CNT concentrations of 5, 8, and 10%, as shown in Figs. 1a-c, respectively.
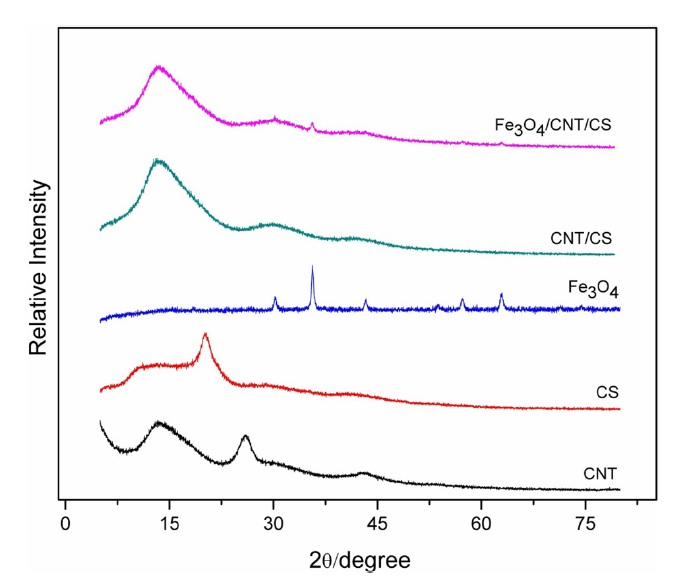
The diffraction patterns of CTS, CNTs, Fe3O4 and the nanocomposite films are shown in Fig. 2. In the diffraction pattern
of CS, one main peak was observed at 2θ = 20° (maximum intensity) corresponding to a characteristic peak of CS chains aligned through intermolecular interactions [19]. The characteristic sharp peak of CNTs at 2θ = 26° represents C (002), which is attributed to the ordered arrangement of concentric cylinders of graphitic carbon in the nanotube [16]. This crystalline peak is not present in the nanocomposite samples, suggesting the dispersion of CNTs into the CS matrix (17). XRD patterns for the Fe3O4 nanoparticles displayed characteristic peaks (2θ = 30.1°, 35.5°, 43.1°, 53.4°, 57.0°, and 62.6°). These peaks are consistent with those found in the Joint Committee on Powder Diffraction Standards (JCPDS) database (PDF No. 65-3107). Patterns for the Fe3O4/CNT/CS composites revealed the presence of such peaks, indicating that the Fe3O4 particles in the composites were pure Fe3O4 with a spinel structure.
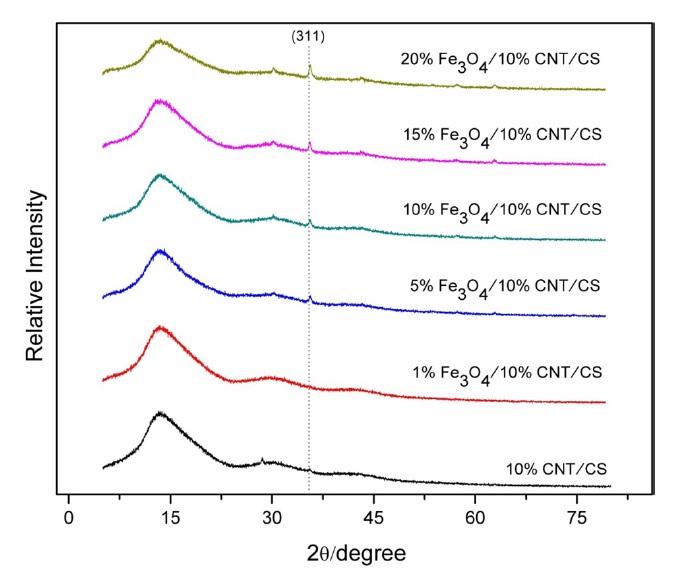
Fig. 3 clearly shows how increasing Fe3O4 loading in the composites resulted in increasing corresponding peak intensities. The figure further shows that neither the CNTs nor CS induced a phase change in Fe3O4. Furthermore the results show how the increase in Fe3O4 concentration broadened the main peaks, specifically the (400) peak above a Fe3O4 to CNT ratio of 1:1, which indicates a higher average particle size of Fe3O4 due to increased agglomeration of the nanoparticles. The average particle size, calculated using Scherrer’s formula, was approximately 30.79 nm and 46.61 nm for the 1:1 and 2:1 ratios of Fe3O4 to CNT, respectively. Hence the decrease in conductivity at higher Fe3O4 to CNT ratios was attributed to the agglomeration of the nanoparticles, which hindered the effectiveness of the conductive channels between CNTs; this consequently reduced the conductivity percolation threshold of the composites.
Fe3O4/CNT/CS nanocomposite films were successfully prepared using a simple solution casting method. A synergistic effect of Fe3O4 and CNTs on the electrical conductivity of the nanocomposite films was observed, where by an optimal loading of Fe3O4 resulted in a ratio of 1:1 relative to the CNT content of the nanocomposite film. XRD patterns revealed that higher Fe3O4 to CNT ratios increased the agglomeration of the Fe3O4 nanoparticles, which hindered the synergistic effect on the conductivity.