Chrysanthemi Flos (CF), the flowers of
Macrophages are main players in the immune function as antigen-presenting cells in initiating adaptive immune responses and in the innate immune response [3]. Many studies have demonstrated that
Much evidence exists that herbs have effective immunom-odulatory activities in both preclinical and clinical resea-rches [9-13], and studies of the modulatory effects on nitric-oxide (NO) production have been conducted [14-16]. Also, medicinal herbs, Compositae, have been studied for immune regulation [17], but few studies have addressed Chrysanyh-emi Flos regulating the production of nitric-oxide (NO). The-refore, the aim of this study was to use the lipopolysacch-aride (LPS)-treated RAW 264.7 murine macrophage model to further explore the potential immunomodulation of CF, especially for the regulation of NO production.
Chrysanthemi Flos was purchased from Omniherb (Korea). CF was prepared as follows: Chrysanthemi Flos, 100 g, was added to 2,000 ml of distilled water and boiled in a heating extractor for 3 hours. The extract was filtered and concentrated by using a rotary evaporator and was lyophilized by using a freeze dryer (17.9 g). The lyophilized extract was dissolved in water and filtered three times through microfilter paper (What-man no. 2, 0.45-0.2
Mouse monocyte/macrophage cell line RAW 264.7 (ATCC TIB-71) was grown in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), streptomycin (100 mg/ml) and penicillin (100 U/ml), at 37℃ with 5% CO2. Cells were transferred to 96-well culture plates at a density of 1 x 105 cells/well and were allowed to adhere for 24 hours prior to sample treatment. The culture supernatant was collected and stored at -80℃ until it was analyzed for nitric-oxide (NO), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-
The general viability of cultured cells was determined by reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) to formazan. MTT in living cells is reduced to formazan crystals, and the amount of formazan dissolved in dimethyl sulfoxide (DMSO), as measured by using the by spectroscopic method, shows the growth of cells. The cell were seeded in 96-well plates at a density of 1 x 105 cells/well and were cultured at 37℃ in 5% CO2. Cells were pretreated with the sample at concentration, of 0, 50, 100 and 200
Cell viability (%) = [(optical Density 595 of sample)/(optical density 595 of control)]×100.
RAW 264.7 cells were seeded in triplicates at a density of 5 x 104 cells/well in 96-well plates with complete DMEM and were allowed to adhere for 24 hours. Cells were cultured with LPS (1
The NO concentration was determined by measuring the amount of released nitrite with Griess reagent (Sigma-Aldrich, Missouri, USA) according to the Griess reaction [19]. Griess rea-gent produced a chemical reaction with nitrite and formed a purple azo salt that was consistent with the NO concentration. Briefly, 50
The concentrations of IL-6 and TNF-
The results were expressed as means ± standard deviations (SD). Significant changes were evaluated by using the one-way ANOVA with Dunnett’s post-hoc test. Values of p < 0.05 were considered significant.
3.1. Cytotoxicity on RAW 264.7 cells
In order to evaluate the cytotoxicity of CF, samples were prepared at various concentrations and used to treat RAW 264.7 cells. The results of this evaluation are shown in Fig. 1 at conce-ntrations of 50, 100 and 200
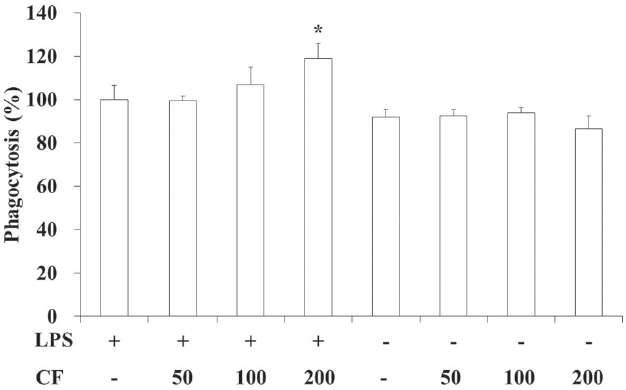
3.2. Phagocytic activity of RAW 264.7 cells
In order to evaluate the phagocytic activity of CF, we prepared samples at various concentrations and used them to treat RAW 264.7 cells. The results of this evaluation are shown in Fig. 2 at concentrations of 50, 100 and 200
Both LPS and CF treatments increased the phagocytic activities compared to LPS treatments in a dose-dependent manner. Especially both LPS and CF (200
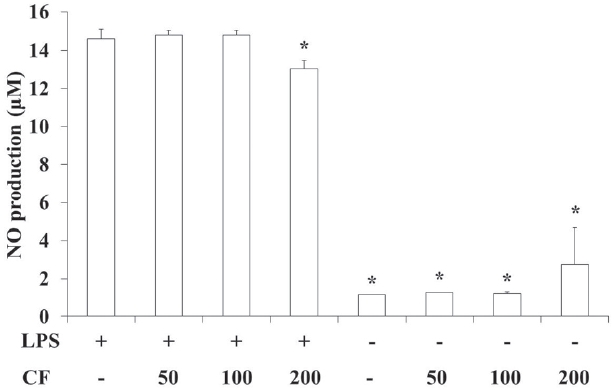
3.3. NO production of RAW 264.7 cells
In order to evaluate the NO production of CF, we prepared samples at various concentrations and used them to treat RAW 264.7 cells. The results of this evaluation are shown in Fig. 3 at concentrations of 50, 100 and 200
LPS treatment significantly increased the NO production compared to vehicle treatment (p < 0.05), but both LPS and CF (200
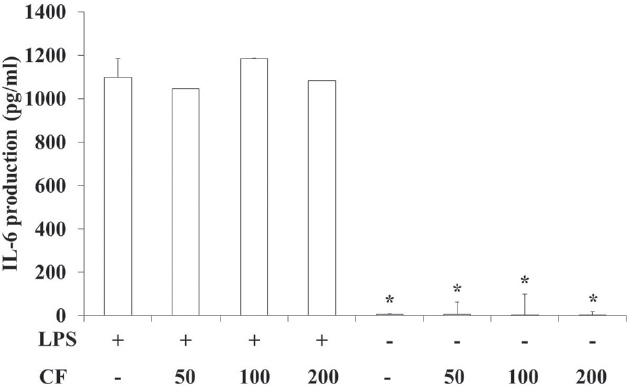
3.4. IL-6 production of RAW 264.7 cells
In order to evaluate the IL-6 production of CF, we prepared samples at various concentrations and used them to treat RAW 264.7 cells. The results of this evaluation are shown in Fig. 4 at concentrations of 50, 100 and 200
LPS treatment significantly increased the IL-6 production compared to vehicle treatment (p < 0.05). However, CF treatme-nt did not change the IL-6 production induced by LPS treatment (Fig. 4).
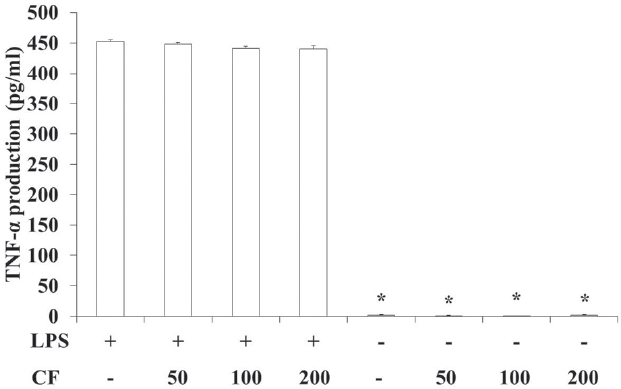
3.5. TNF-α production of RAW 264.7 cells
In order to evaluate the TNF-
LPS treatment significantly increased the TNF-
Chrysanthemi Flos, the flowers of
LPS is present in the cell's outer membrane of gram negative bacteria and augments pro-inflammatory cytokines, such as TNF-
LPS as endotoxin [23] causes an immune response, but excessive production of inflammatory mediators can destroy normal tissues and cause shock [24]. Thus, herbs that regulate the immune response are important. Many studies concerning the immunomodulatory effects of herbs, such as immunomodu-latory effects on healthy volunteers, anti-tumor immune response, splenocyte proliferation, macrophage function, NK anti-tumor activities and so on [9-13], have been performed.
To investigate the in this study, CF on LPS-stimulated RAW 264.7 macrophages were examined immunomodulatory effects by measuring the cytotoxicity, the phagocytic activity and the amount of NO, IL-6 and TNF-
NO is generated by macrophages as part of the human immu-ne response. The immune system may regulate the armamen-tarium of the phagocytes that play a role in the inflammation and the immune responses with NO. NO secreted as an immune response acts as a free radical and is toxic to bacteria. The mechanisms for this include DNA damage [26-28] and degrada-tion of iron-sulfur centers into iron ions and iron-nitrosyl compounds [29]. NO shows an anti-cancer or an anti-microbial effect, but excessive secretion can kill normal cells or promote inflammation [30]. In this study, LPS treatment significantly increased the NO production compared to vehicle treatment (p < 0.05). However, both LPS and CF (200
IL-6 is an interleukin that acts as both a pro-inflammatory and an anti-inflammatory cytokine. It is secreted by macrophages and T cells to stimulate immune response, e.g., during infection, but excessive secretion causes rheumatoid arthritis and inflammatory diseases, such as Crohn's disease [31]. TNF-
LPS treatment significantly increased the IL-6 and the TNF-
In conclusion, CF regulates the production of NO and can prevent the pathological phenomena caused by excessive production of NO, such as expansion of blood vessels, mutation and damage to organization and nerve by inflammation. CF may play an important role in intensity modulated NO-induced immune responses and may have potential for use as an immunoenhancing pharmacopuncture. Further studies will be needed to unravel the characteristics of the effects of CF and the mechanisms of its functions.