Many efforts have shown multi-oncologic roles of galectin-3 for cell proliferation, angiogenesis, and apoptosis. However, the mechanisms by which galectin-3 is involved in cell proliferation are not yet fully understood, especially in human colon cancer cells.
To cluster genes showing positively or negatively correlated expression with galectin-3, we employed human colon cancer cell lines, SNU-61, SNU-81, SNU-769B, SNU-C4 and SNU-C5 in high-throughput gene expression profiling. Gene and protein expression levels were determined by using real-time quantitative polymerase chain reaction (PCR) and western blot analysis, respectively. The proliferation rate of human colon cancer cells was measured by using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay.
Expression of γ-aminobutyric acid B receptor 1 (GABABR1) showed a positive correlation with galectin-3 at both the transcriptional and the translational levels. Downregulation of galectin-3 decreased not only GABABR1 expression but also the proliferation rate of human colon cancer cells. However, Korean herbal extract, HangAmDan-B (HAD-B), decreased expression of GABABR1 without any expressional change of galectin-3, and offset γ-aminobutyric acid (GABA)-enhanced human colon cancer cell proliferation.
Our present study confirmed that GABABR1 expression was regulated by galectin-3. HAD-B induced galectin-3-independent down-regulation of GABABR1, which resulted in a decreased proliferation of human colon cancer cells. The therapeutic effect of HAD-B for the treatment of human colon cancer needs to be further validated.
Galectin-3 is a member of the family of
Many studies have shown that galectin-3 regulates cancer cell proliferation. Galectin-3-stimulated cell proliferation of IMR-90 human lung fibroblasts [6]; a decrease of galectin-3 expression in activated T lymphocytes paralleled a downregulation or even a blocking of proliferation [9]; and the introd-uction of galectin-3 cDNA caused human lymphoma Jurkat T cells to grow faster [10]. A recent report provided evidence that downregulation of galectin-3 led to diminished human colon cancer cell proliferation via modulation of the hete-rogeneous nuclear ribonucleoprotein Q (hnRNP Q) level [11].
Overexpression of galectin-3 has been reported in gastric cancer [12]. Positive galectin-3 expression was observed in 84% of gastric cancer cases. In enhanced cells of a cancerous lesion, 48% showed stronger nuclear immunoreactivity than a cytoplasmic one whereas adjacent epithelial cells showed little or weak nuclear immunoreactivity [12]. In addition, decreased galectin-3 expression was found in breast [13], ovary [14], prostate [15], epithelial skin cancer [16], and head-and-neck squamous cell carcinomas [17] than in corresponding normal tissue.
HangAmDan (HAD)-B consists of eight species of Korean medicinal plants and animals (Table 1), and is an upgraded version of HangAmDan (HAD) used traditionally for solid masses, which also shows anti-angiogenic activity [18]. A mixture of these plants has been shown to exert strong anticancer activity against solid tumors, including pancreatic, lung, colorectal, and stomach cancers. Additionally, anti-angiogenesis effects and inhibition of cancer cell proliferation and metastasis have been reported [19]. In particular, case reports observed with HAD have been selected as part of the National Cancer Institute’s Best Case Series Program [20]. HAD-B has shown efficacy in inhibiting migration and proliferation of human umbilical vein endothelial cells and in limiting the formation of capillary tube structures [21]. Furthermore, a safety evaluation of HAD-B has revealed no side-effects in both healthy subjects and cancer patients [22].
Even though a number of studies have reported the functions of galectin-3 in many types of cancer, the mechanisms by which galectin-3 is involved in cell proliferation are not yet fully understood, especially in human colon cancer cells. In the present study we report that
2.1. Human colon cancer cell lines
Human colon cancer cell lines, SNU-61, SNU-81, SNU-769B, SNU-C4 and SNU-C5, were obtained from the Korean Cell Line Bank (Seoul, Korea).
2.2. Preparation of water extract of HAD-B
HAD-B was provided from the East-West Cancer Center of Dunsan Oriental Medical Hospital, Daejeon University, Daejeon, Korea (Table 1). The water extract of HAD-B was prepared by extracting HAD-B powder with 10-times (v/w) the amount of distilled water at room temperature for 24 hrs. The extract was centrifuged at 1000×g for 30 mins and was then filtered and lyophilized. The extract powder was dissolved directly in distilled water.
2.3. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
A colorimetric assay using tetrazolium salt, MTT, was used to assess cell proliferation after galectin-3 suppression. MTT assays were performed as described in a previous report [11]. Briefly, equal numbers of cells were incubated in each well in 0.18 ml of culture medium to which 0.02 ml of 10 × 5-FU (Choongwae Pharma Corporation), HAD-B, GABA or PBS (for untreated 100% survival control) had been added. After 4 days of culture, 0.1 mg of MTT was added to each well and incubated at 37℃ for a further 4 hrs. Plates were centrifuged at 450 × g for 5 mins at room temperature, and the medium was removed. Dimethyl sulfoxide (0.15 ml) was added to each well to solubilize the crystals, and plates were immediately read at 540 nm by using a scanning multiwell spectrometer (Bio-Tek Instruments Inc., Winooski, VT). All experiments were performed three times, and the IC50 (
Western blot analyses were performed as described in a previous report [11]. Primary antibodies against galectin-3 (Abcam, Cambridge, UK),
All procedures were performed at 4℃ unless otherwise specified. Approximately 107 cells in 1 ml of cold 1 × radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (Roche Diagnostics) were incubated on ice for 30 mins with occasional mixing. Cell lysates were centrifuged at 12,000 × g for 10 mins, and the supernatant was collected carefully without disturbing the pellet. The supernatant was mixed with primary antibody against either galectin-3 (Abcam) or GABABR1 (Abcam) and was incubated for 2 hrs on a rocking platform. Prepared protein G sepharose beads (GE Health Care Life Sciences) were added and further incubated on ice for 1 hrs on a rocking platform. The mixture was centrifuged at 10,000 g for 30 s, and the supernatant was removed completely. Protein G sepharose beads were washed 5 times with 1 ml of cold 1 × RIPA to minimize the background. Next, 100
2.6. Intracellular cAMP measurement
The intracellular cAMP for human colon cancer cells was determined by using a cAMP Direct Immunoassay Kit (Abcam), as recommended by the manufacturer.
2.7. RNA preparation and Affymetrix GeneChip hybridization
Total RNA was extracted using Trizol reagent (Life Technologies, Inc., Carlsbad, CA), according to the manufacturer’s instructions. Genes expressed in the chemosensitive and chemoresistant groups were analyzed on a high-density oligonucleotide microarray (HG-U133A; Affymetrix, Santa Clara, CA) containing 22,283 transcripts. Target preparation and microarray processing procedures were performed, following the Affymetrix GeneChip Expression Analysis Manual (Affymetrix). Briefly, total RNA extracted was purified with an RNeasy kit (Qiagen). Double-stranded cDNA was synthesized from total RNA (20
2.8. Affymetrix GeneChip data analysis
A GeneChip analysis was performed based on the Affymetrix GeneChip Manual (Affymetrix) with Data Mining Tool (DMT) 2.0 and Microarray Database software. All genes represented on the GeneChip were globally normalized and scaled to a signal intensity of 500. Fold changes were calculated by comparing transcripts between the cell lines tested. The DMT 2.0 software employed changed calls (increased or decreased) to analyze the expression of a particular transcript statistically and to determine whether it had been relatively increased, decreased or remained unchanged. After filtration through a "present" call (
2.9. Real-time quantitative reverse transcription polymerase chain reaction
Four genes (ELF3, AXIN2, ENO2 and SACS) were selected for real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) for validation of the microarray data. Using the SuperScript Pre-amplification System for first strand cDNA synthesis, 5 mg of total RNA was used for creation of singlestranded cDNA (Life Technologies). The cDNA was diluted and quantitatively equalized for PCR amplification. For real-time
qRT-PCR, the ABI Prism 7900 sequence detection system (Applied Biosystems) was used. AccuPower GreenStar PCR Master Mix (Bioneer Corporation, Daejeon, Korea) was used for each PCR reaction, and the GAPDH gene was simultaneously run as a control and was used for normalization. Non-template-control wells without cDNA were included as negative controls. Each test sample was run in triplicate. The primer sets for PCR amplification were designed as follows: ELF3-F: 5’-TGAGCTGCTGGAGAAGGATG-3’, ELF3-R: 5’-CCCTTCTTGCAGTCACGAAA-3’, AXIN2-F: 5’-AATCATTCGGCCACTGTTCA-3’, AXIN2-R: 5’-CACAGGCAAACTCATCGCTT-3’, ENO2-F: 5’-CTGATGCTGGAGTTGGATGG-3’, ENO2-R: 5’-CCATTGATCACGTTGAAGGC-3’, SACS-F: 5’-CCATTTGTTGGCATTTTTGG-3’, and SACS-R: 5’-CGCTCATGTTTCAGTGCCTT-3’. Following the standard curve method, the expressed quantities of the examined genes were determined using the standard curves and the CT values and were normalized using the GAPDH expression quantities.
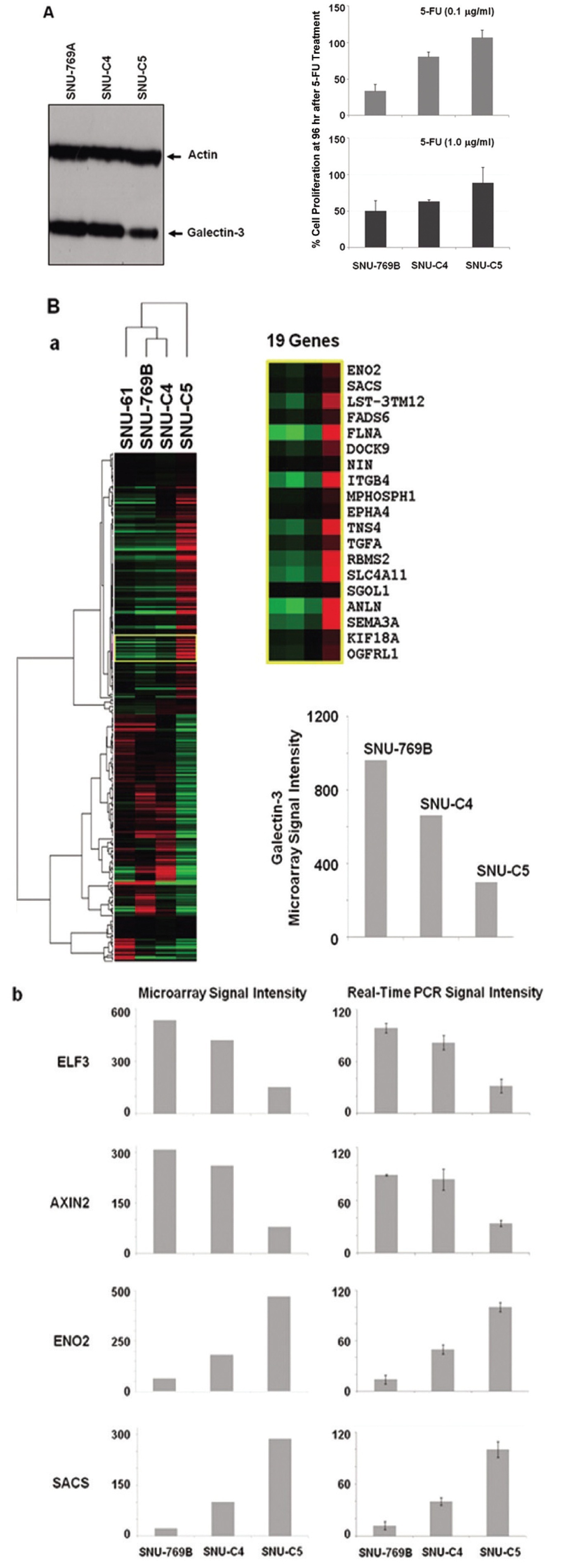
3.1. Galectin-3 expression related to 5-FU susceptibility in human colon cancer cells
To confirm the correlation between galectin-3 expression and 5-FU susceptibility in human colon cancer cells, we performed Western blot and MTT analyses on three human colon cancer cell lines, SNU-769B, SNU-C4 and SNU-C5. 5-FU susceptibility showed a decreasing tendency that depended on both the transcriptional (Fig. 1A) and the translational (Fig. 1Ba) levels of galectin-3. To cluster the genes showing positively or negatively correlated expression with galectin-3, we employed SNU-61, which had almost the same 5-FU susceptibility as SNU-769B, in a high-throughput gene expression profiling experiment (Fig. 1B & Tables 2, 3). Figure 1Ba shows an example of 19 genes clustered in a galectin-3 expression pattern, which was confirmed by real-time PCR (Fig. 1Bb). The top 50 down- and up-regulated genes in SNU-C5, compared to SNU-769B, are listed in Tables 2 and 3, respectively.
Although the genes listed in Tables 2 and 3 contain
3.3. Galectin-3-independent down-regulation of GABABR1 protein by HAD-B in human colon cancer cells
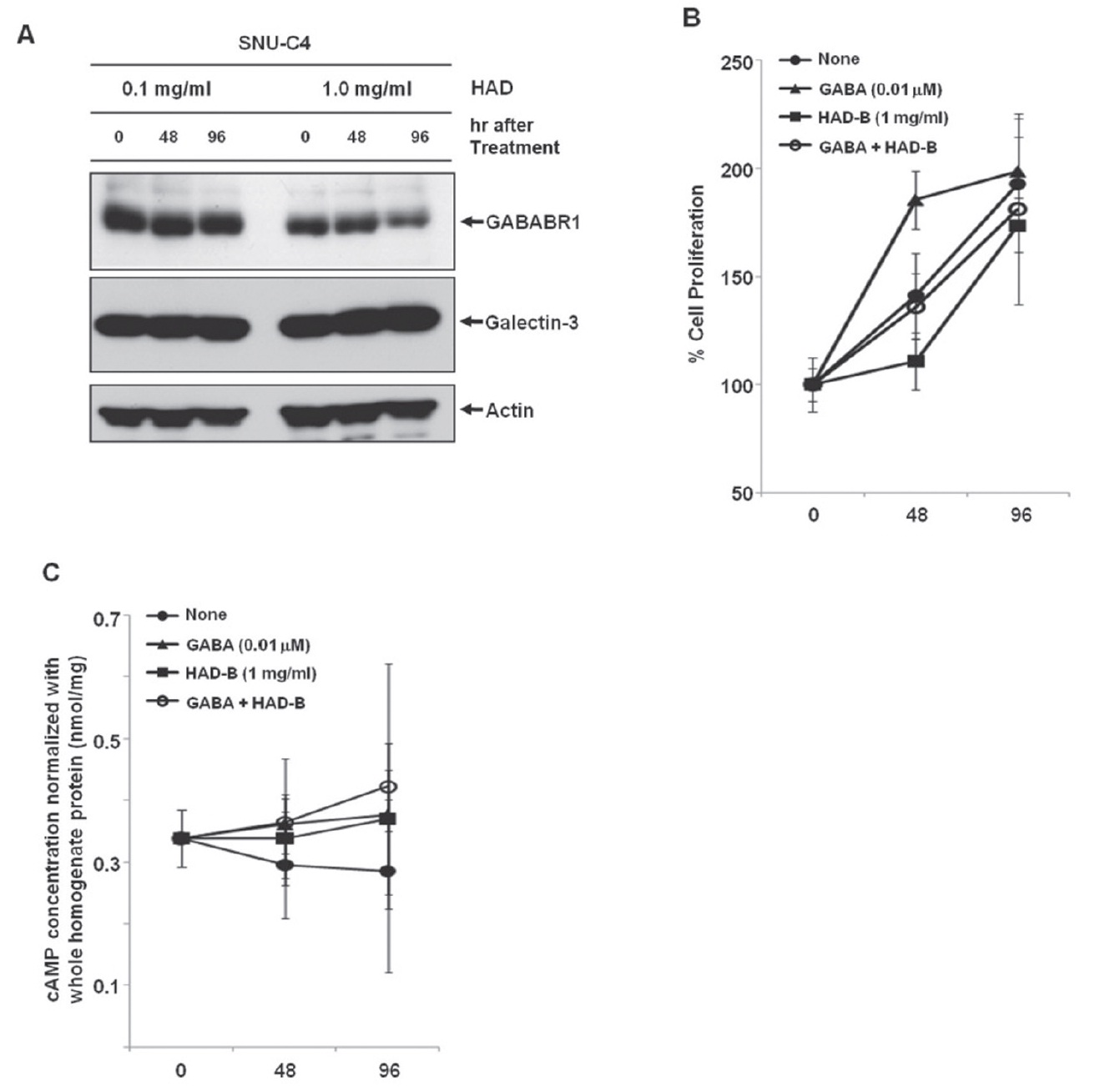
To check the effect of HAD-B treatment on the expression level of galectin-3 and GABABR1, we cultured SNU-C4 with modest expression of galectin-3 and GABABR1 in the presence of HADB, and we performed a Western blot analysis. At 96 hrs after treatment with 1 mg/ml HAD-B, expression of GABABR1 was reduced, but galectin-3 did not show any expressional change (Fig. 3A).
3.4. GABABR1-mediated proliferation of human colon cancer cells suppressed by HAD-B treatment
Treatment with
maintained (Fig. 3B). HAD-B significantly decreased cell proliferation at 48 hrs after treatment compared to the control, but the suppressed proliferation had recovered at 96 hrs (Fig. 3B). Cells co-treated with GABA and HAD-B showed almost the same pattern of proliferation as that of the control (Fig. 3B). Either GABA or HAD-B treatment slightly increased the intracellular cAMP in SNU-C4 compared to that in the nontreated control (Fig. 3C).
Colon cancer causes almost a half million deaths every year [23]. In the past 3 decades, 5-fluorouracil (5-FU) chemotherapy and 5-FU-based chemotherapy have been the mainstream in adjuvant treatment of colon cancer [24]; however, partial or complete responses of colon cancer to 5-FU are generally followed by eventual tumor re-growth [25]. Numerous studies have focused on identifying the mechanisms and key molecules involved in natural or acquired 5-FU resistance. Nevertheless, conclusive and consistent results have not been obtained so far. A recent proteome approach identified galectin-3 as a protein affecting 5-FU resistance and the proliferation rate of human colon cancer cells [11]. Our present study confirmed the correlation between galectin-3 expression and 5-FU susceptibility in three human colon cancer cell lines. 5-FU susceptibility of human colon cancer cells was different depending on both the transcriptional and the translational levels of galectin-3 (Figs. 1A and B). Because the identification of genes showing positively or negatively correlated expression with galectin-3 can provide further information on how galectin-3 regulates proliferation of human colon cancer cells, a high-density oligonucleotide microarray was performed. From this transcriptional analysis, we were able to list the genes down- and up-regulated based on the level of galectin-3 expression (Fig. 1B, Tables 2 and 3). Though
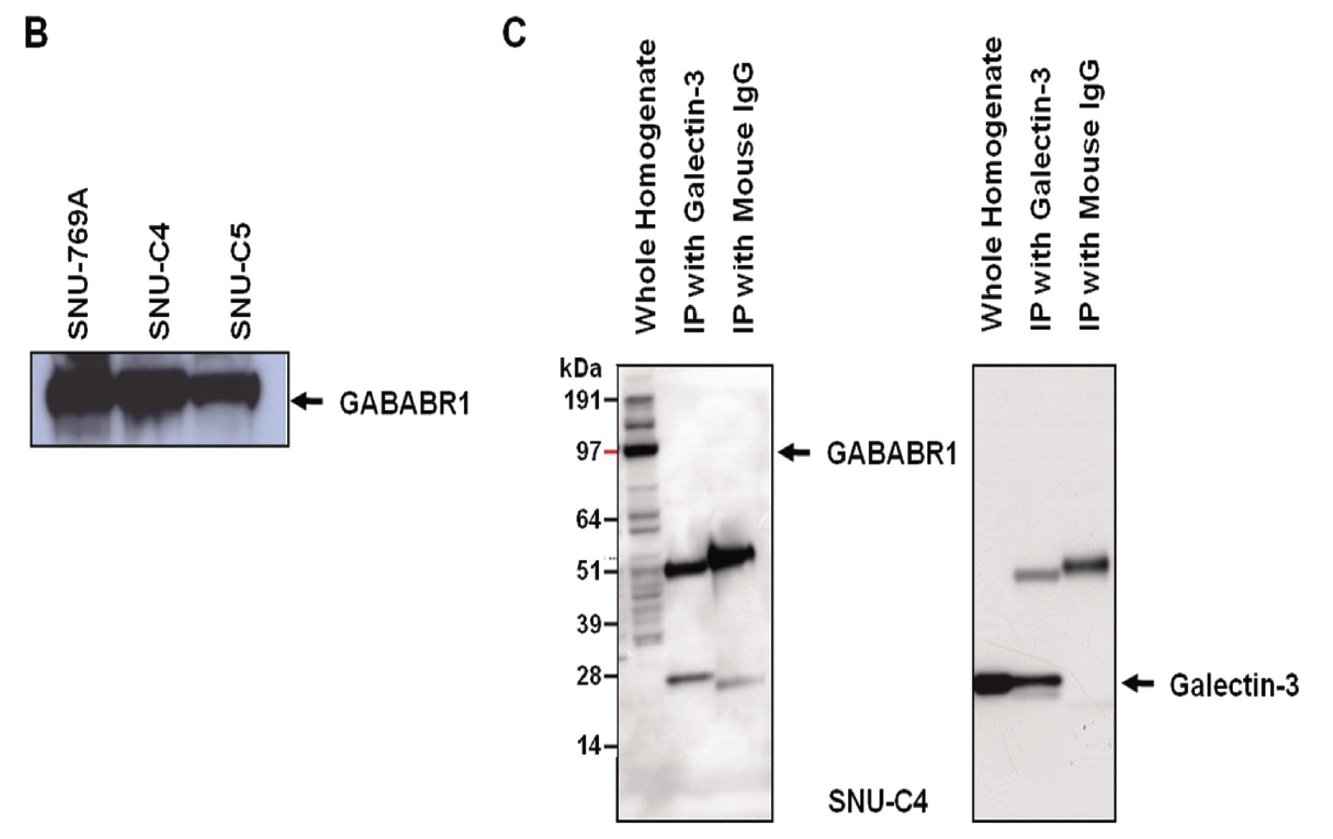
GABABRs have been found to play a key role in regulating membrane excitability and synaptic transmission in the brain [26]. GABABRs are G-protein coupled receptors that associate with a subset of G-proteins that trigger cAMP cascades [26]. GABABR subtypes exist; two GABAB-receptor splice variants, GABABR1a and GABABR1b, have been cloned [27], and a new GABABR subtype, GABABR2, does not bind with available GABAB antagonists with measurable potency [28]. GABABR1a, GABABR1b and GABABR2 alone do not activate Kir3-type potassium channels efficiently, but co-expression of these receptors yields a robust coupling to activation of Kir3 channels. GABABR2 and GABABR1a/b proteins immunoprecipitate and localize together at dendritic spines [28]. The heteromeric receptor complexes exhibit a significant increase in agonist- and partial agonist-binding potencies as compared with individual receptors and probably represent the predominant native GABAB receptor [28]. As a previous report also showed that the transcriptional level of GABABR1 was decreased by transfection of galectin-3 small interfering RNA (siRNA) [11], expression of GABABR1 could be regulated by galectin-3. However, reverse immunoprecipitation to validate the interaction between two proteins revealed that galectin-3 did not affect the protein stability of GABABR1 because it formed a complex with GABABR1 (Fig. 2C).
Gamma-aminobutyric acid (GABA) has been reported to affect cancer development. For example, GABA can be a potential tumor suppressor for small airway-derived lung adenocarcinomas [29]. The GABA agonist nembutal has been reported to be a potent inhibitor of primary colon cancer and metastasis [30]. The GABABR agonist baclofen induced G(0)/G(1) phase arrest of human hepatocellular carcinomas (HCCs), which suggested the possibility of developing baclofen as a therapeutic drug for the treatment of HCCs [31]. Furthermore, stimulation of GABABR signaling has been suggested as a novel target for the treatment and the prevention of pancreatic cancer [32]. However, in our present study, treatment with GABA promoted proliferation of the human colon cancer cell line SNU-C4 (Fig. 3B). The Korean herbal extract HAD-B not only decreased GABABR1 expression but also reduced proliferation of human colon cancer cells without any expressional change of galectin-3 (Figs. 3A and 3B). GABABR activation can lead to down-regulation of the intracellular cAMP level in human cancer cells [30,32]. Downregulation of GABABR1 by HAD-B treatment increased the basal level of intracellular cAMP in SNU-C4 (Fig. 3C). However, such an increased cAMP was also observed after GABA treatment (Fig. 3C). The overall findings in the present study were inconsistent with those in previous reports describing the activation of GABABR1 to prevent the progression of a human carcinoma. Nevertheless, our present results showed a link between galectin-3 and GABABR1 in human colon cancer cell proliferation. Galectin-3 regulated GABABR1 expression [11]. Decreased galectin-3 expression reduced not only GABABR1 expression but also the proliferation rate of human colon cancer cells [11]. Even GABA promoted human colon cancer cell proliferation by activating GABABR1 signaling, and the increased proliferation was offset by HAD-B treatment because HAD-B led to galectin-3-independent down-regulation of GABABR1 (Fig. 3).
Our present study confirmed that GABABR1 expression was regulated by galectin-3. Korean herbal extract HAD-B induced galectin-3-independent down-regulation of GABABR1, which resulted in a decreased proliferation of human colon cancer cells. The therapeutic effect of HAD-B for the treatment of human colon cancer needs to be further validated.