Eye drops are a medical treatment applied to various opht-halmological diseases, so they must be safe and non-irritati-ng as they directly affect the eyes. Especially, the fact that no blood vessels exist in the cornea makes localized eye-dropping more available and effective than oral medication;besides, effective cleansing of bacteria and toxins can be mostly achieved through the proper use of eye drops [1]. However, recently there have been no standardized pharm-aceutical companies manufacturing eye drops for clinical use in korean medicine. For that reason, many pharmacopunctures manufactured by the Korea Pharmacopuncture Institute, which has aseptic facilities almost equivalent to the GMP level, are considered to be the most appropriate mate-rials for making eye drops.
In order to utilize Pharmacopuncture solutions (PSS) from the Korean Pharmacopuncture Institute as eye drops, there have been constant experimental trials on ascertaining the safety and the effectiveness of saline solution,
2.1.1. Samjeong pharmacopuncture solution
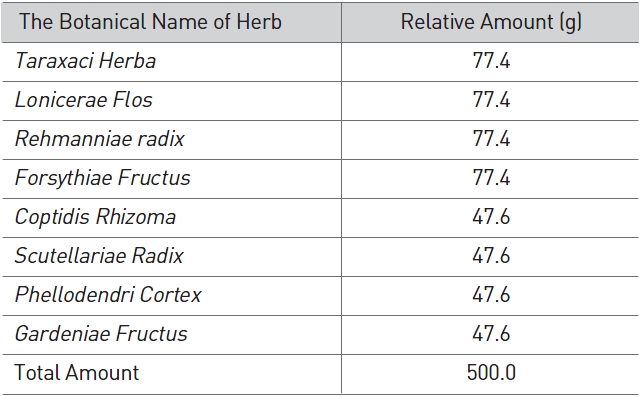
SPS used in this study was manufactured using a low-temper-ature extracting process and was provided by the Korea Pharm-acopuncture Institute. The prescription is given in Table 1.
[Table 1] Prescription of Samjeong
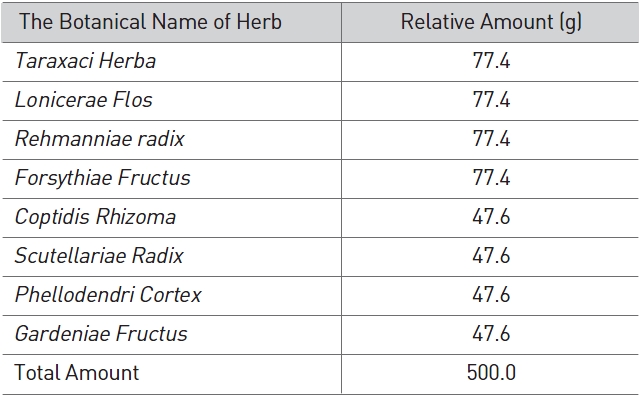
Prescription of Samjeong
2.1.2. Animals
In this study, animal experiments were conducted under the approval of the Pusan National University Hospital Institutional Animal Care and Use Commitee
Rabbits were bred in the rabbit cage (420W×500D×310H ㎜) made of stainless and had free access to feed (Sinchon Co.) and water. The environment was maintained at a constant tempera-ture (21±2℃) and humidity (60%).
2.1.3. Cell line and culturing strain
The cell line used in this experiment was provided by Korean Collection for Type Culture (KCTC).
2.2.1. Eye irritation test
Eye irritation tests were conducted following the toxicity tes-ting regulation of the Korea Food & Drug Administration (2009.8. 24, KFDA 2009-116). Both eyes of all the laboratory rabbits had been tested 24 hours before the experiment began, among which rabbits with normal corneas were selected. SPS, 0.1 ㎖ was dropped on an eye of each of the nine rabbits, and after 20∼30 seconds, three out of these were washed with 20 ㎖ of warm saline solution for a minute while the others were left untouched to be treated as a control group. After the application of SPS, changes in the weight and clinical symptoms on the 1st, 2nd, 3rd, 4th and 7th day were observed. The eye irritation tests were evaluated, with the maximum points set at 80 points on cornea response, 10 points on iris response and 20 points on the conjunctiva response, adding up to a total score of 110. If injury still remained on the affected eyes afterwards, the eye-dropping was applied every three days over 13 days (Tables 2, 3, and 4).
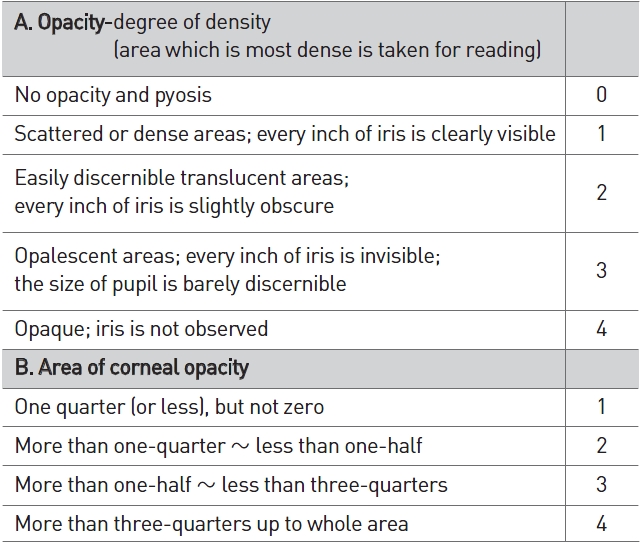
[Table 2] Scale of weighted scores used for grading the severity of ocular lesions (cornea)
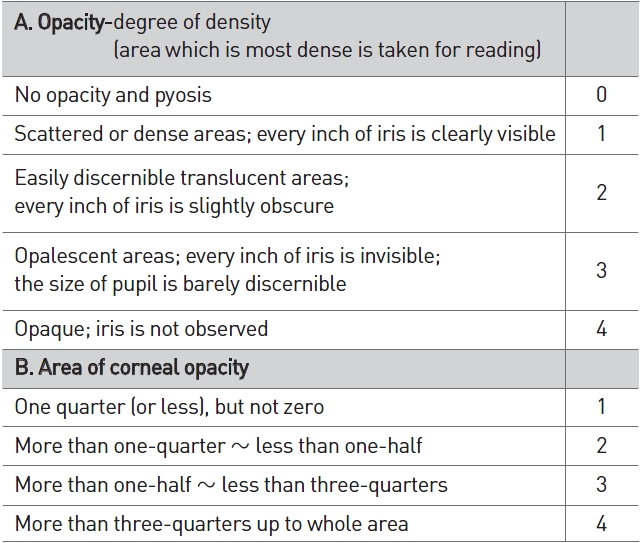
Scale of weighted scores used for grading the severity of ocular lesions (cornea)
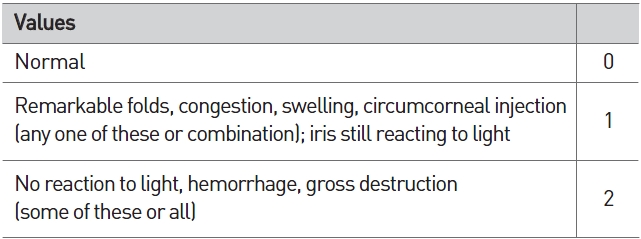
[Table 3] Scale of weighted scores used for grading the severity of ocular lesions (iris)
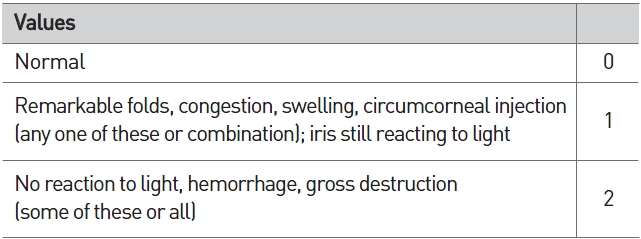
Scale of weighted scores used for grading the severity of ocular lesions (iris)
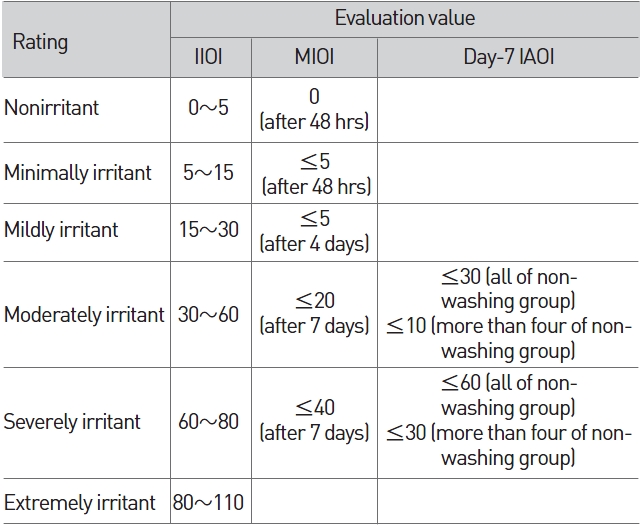
"The mean index of ocular irritation (MIOI)" which had been obtained from the division of the total score of "the individual index of ocular irritation (IIOI)" by the number of rabbits, "the index of acute ocular irritation (IAOI)", which is the maximum value of "the mean index of ocular irritation (MIOI)" during observation and the day-7 IOII (indivisual ocular irritation index) were used in order to evaluate the degree of eye irritability (Table 5).
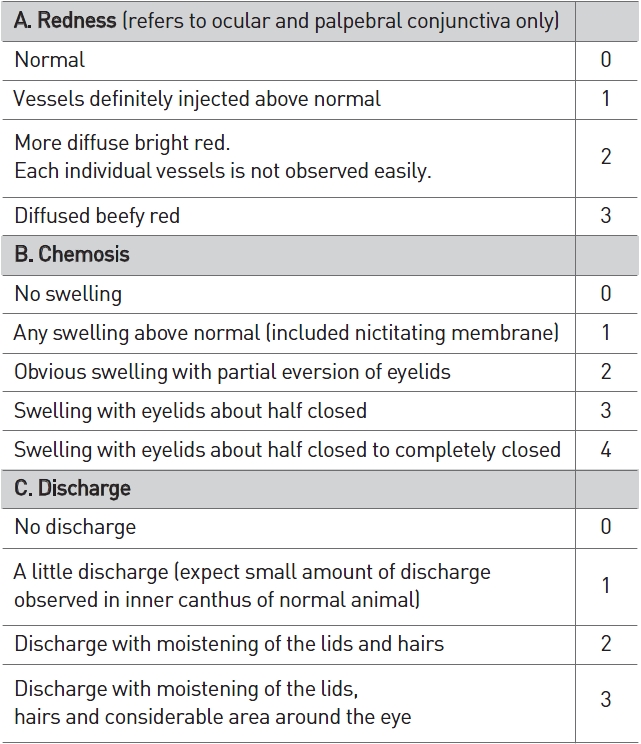
[Table 4] Scale of weighted scores used for grading the severity of ocular lesions (conjunctiva)
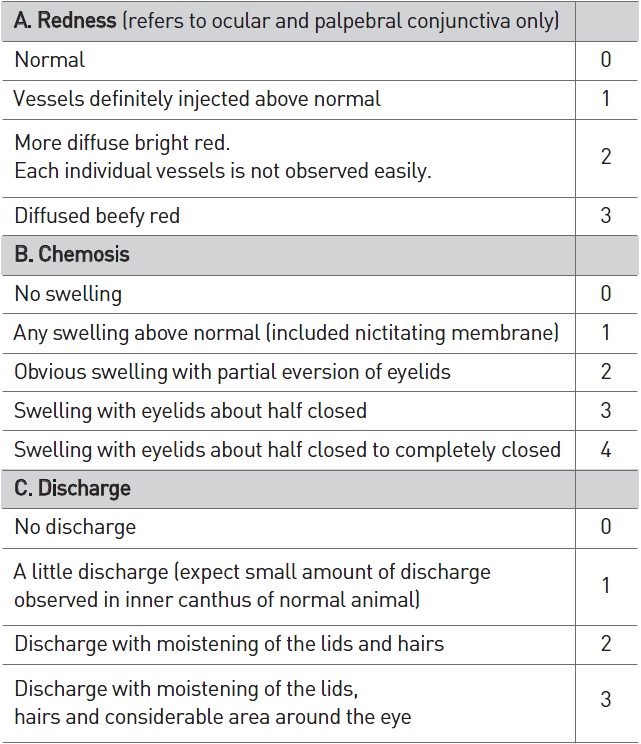
Scale of weighted scores used for grading the severity of ocular lesions (conjunctiva)
[Table 5] Irritation index of eye irritation
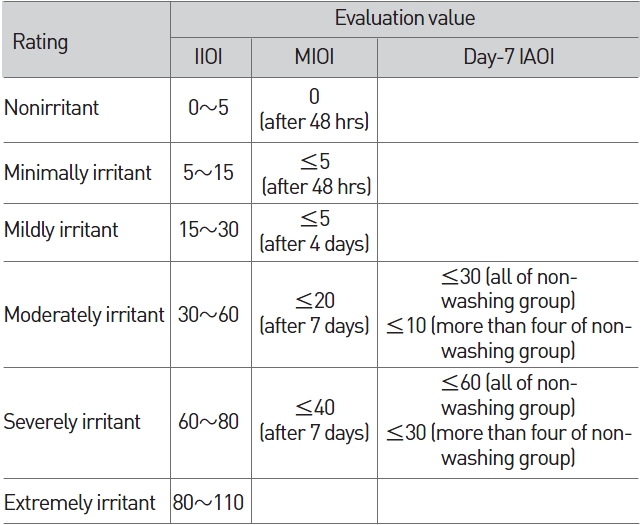
Irritation index of eye irritation
2.2.2. Antibacterial test (filter disc method)
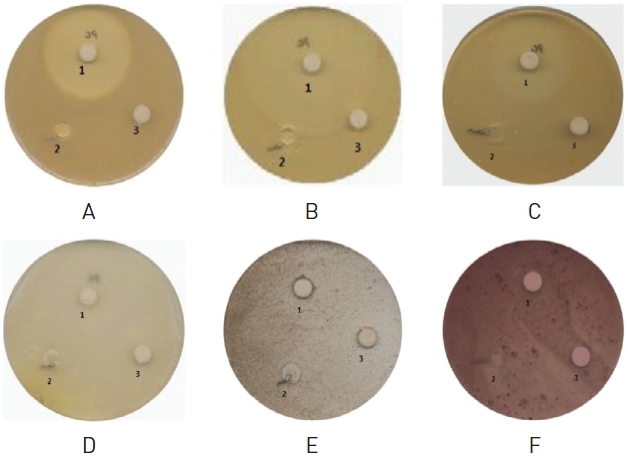
Sterile water was added to the lyophilized ampule, and the medium was coated with 1∼2 droplets of the cell line for 16∼24 hours under 35±1℃. This single colony was then moved to the new medium and subcultured for another 16∼24 hours under 35±1℃. A yeast fungus was cultured for 3 days and a mold was cultured for a week. Several colonies separated from the 10 ㎖ sterile saline solution were suspended, and the number of germs in the colony was counted up to 2.5∼10×109 cells/㎖ through a microscope in order to be used as a source of inoculum. Mold was made to 2.5∼10×109 cells/㎖ in the saline solution with 0.03% detergent and was used as the inoculum source. The prepared inoculum sources were coated with 0.4 ㎖each on the medium and left to dry for 2∼3 minutes with a plate lid slightly open on a clean bench. Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa were cultured in Tryptic soy agar, Candida albicans was cultured in Yeast malt agar, Aspergillus niger was cultured in Malt extract agar, and Fusarium oxysporum was cultured in Potato Dextrose Agar. SPS, 50 ㎕ of sample was dropped on the germ-seeded medium with a sterilized filter disc on top of it. Equal amounts of sterile water and Vioflox (Ofloxacin) were used for negative and positive control, respectively. The antibiotic potency was examined by measuring the clear zone after culturing (2∼7days) each cell line at an appropriate temperature. To measure the minimal inhibitory concentration (MIC), we difuted an appropriate amount of the sample, and we performed the experiment in exactly the same way as above.
3.1. Weight and general conditions
No abnormalities related to general conditions like weight, ap-pearance, feed, water consumption, tremor, spasm, diarrhea, coma, drowsiness, contraction and dilatation of pupils, feces and urine, and disposal per day were found during this experim-ent (Tables 6 and 7).
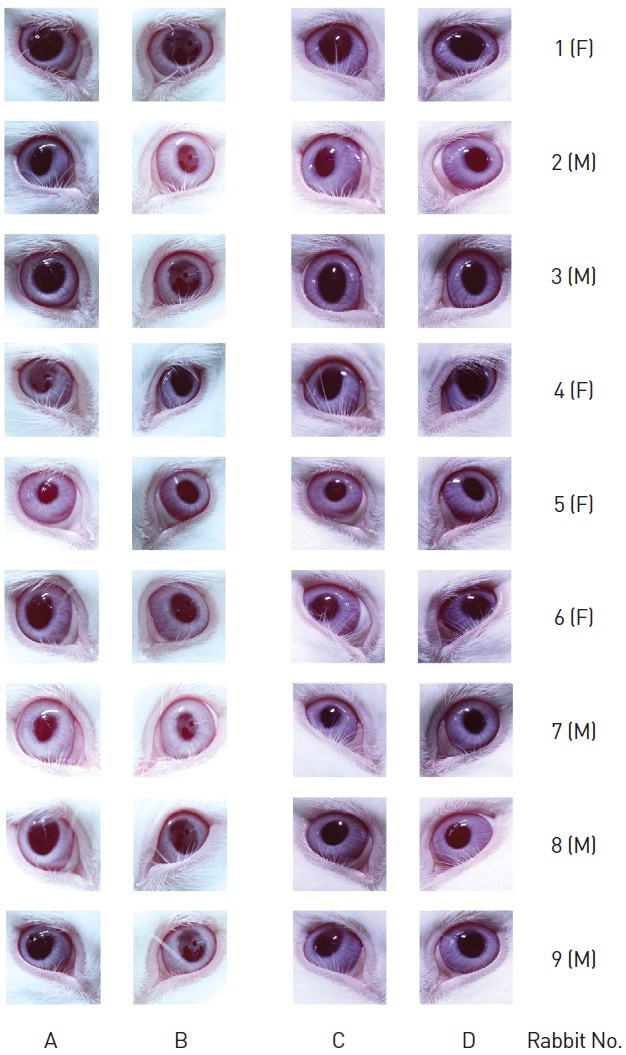
After SPS had been administered only on the left eyes, no eye irritation on the affected cornea, iris and conjunctiva of the nine rabbits from both the washed and the non-washed groups was observed compared with the right eyes (control group) (Table 8, Fig.1).
SPS didn|t show any antibacterial effects on Staphylococcus aureus,
Currently, korean medical doctors are having difficulty treating ophthalmological patients because no standardized pharmace-utical company manufacture eye drops, which can possibly lead to a scale-down in our medical treatment range. Therefore, rea-dily available forms of eye drops desperately need to be immed-iately developed in korean medical ophthalmology as various kinds of eye drops are already prevalent in western medicine. Especially, aseptic forms of eye drops are needed.
PSS is an aseptic treating material that is obtained from single or compound herbs through various extract on methods, and is applied in many clinical fields, chiefly by injection, for musculos-keletal or internal problems. However, the range of korean me-dical ophthalmological treatments could be expanded through this new method to aseptically manufacture PSS for eye drops.
To utilize PSS as eye drops, several experiments have chiefly focused on proving the safety and the effectiveness of anti-infl-ammatory
ammatory herbs [2-8], and "A clinical case report of
In low-temperature extraction, compound herbs are decocted and then separated to undergo decompression and low-temperature distillation. The outside temperature is maintained at 120℃, and the inside temperature is maintained at 60℃ dur-ing decompression and distillation. This method is recommend-ed to maximize the efficacy of herbs and will replace the distill-ation method sooner or later [10].
Thus, by using SPS obtained from low-temperature extraction, we performed an antibacterial activity experiment on eye irritation by using six kinds of infectious keratitis-causing cell lines:
In the antibacterial activity experiment, 50 ㎕ of SPS showed no antibacterial effects on the six kinds of cell lines with infectious keratitis-causing germs, yeast fungus and mold. Even an increased amount up to 200 ㎕ showed no such effects.
SPS is a non-toxic and non-irritant medicine which does not cause any of eye irritation in rabbits, but it has no antibacterial effects on bacterial species that are well known to cause keratitis. Consequently, the fact that SPS causes no eye irritat-ion secures its safety, but the lack of effectiveness in antibacter-ial activity indicates that constant research on a new extract on method is still needed.