Ginseng (
Cultivated ginseng (CG) is cultivated artificially and accounts for the majority of ginseng in the current market. Mountain wild ginseng (WG) grows in natural environments, vegetating in deep mountains, and mountain cultivated ginseng (MCG) can be considered as a mimicry of WG as it is seeded and grown in forests and mountains. WG is considered to be superior to CG, and it has been shown to contain higher levels of ginsenoside, although the reported differences in the total ginsenoside content between WG and CG [4]. Ginsenoside levels were consistently lower in ginseng grown in more intensively cultivated gardens, but growth was consistently higher [5]. In both Korea and China, WG is widely accepted to be more active than CG in chemoprevention. However, little has actually been reported on the differences between WG and CG. Also, the lack of quality control has led to chaos in market distribution [6-7]. Thus, our research team conducted a study to identify WG specific genes for standardization, and we succeeded inidentifying a novel clone, the NRT2 gene, that is a high-affinity nitrate transporter [8].
The technique of suppressive subtractive hybridization (SSH) is believed to generate an equalized representation of differentially expressed genes and to provide a high enrichment of differentially expressed mRNA [9]. SSH overcomes the limitations of other gene analysis methods for differential expression. Its polymerase chain reaction (PCR)-based approach allows for the effective removal of common genes from the RNA population prior to creating the library and has the advantage that reverse transcriptions are amplified efficiently [10]. Chloroplast genomes from several plant species have been sequenced, and have revealed
2.1. Various ginsengs for RNA isolation
The CGs used in this experiment were 6 years of age and were from various regions in Korea. The WGs were 20 to 40 cm long with masses of 20-30 g and age of 30~50 years. These samples were collected from Changbai Mountain in 2008.
2.2. Total RNA isolation and mRNA purification
Ginseng was ground in liquid nitrogen by using a mortar and pestle, and RNA was isolated using the RNeasy Plant RNA Isolation Kit (Qiagen). The concentration of isolated RNA was estimated by measuring its absorbance at 260 nm. An aliquot of the RNA extract was treated with DNase-I (Invitrogen) prior to cDNA synthesis by using Superscript III reverse transcriptase (Invitrogen) and random hexamers according to the manufacturer's protocol.
2.3. Suppressive subtractive hybridization (SSH)
Suppressive subtractive hybridization (SSH) was performed using the Clontech PCR-SelectTM cDNA Subtraction Kit (Clontech) according to the manufacturer’s protocol. The SSH method includes six steps (cDNA synthesis, RsaI digestion and adaptor ligation, two rounds of hybridization and two types of PCRs) for isolating differentially expressed genes. The cDNA fragments, derived from the SSH forward subtractive library (tester: WG; driver: CG), were cloned in pEC-T vector (KOMA Co., Seoul, Korea). The positive clones containing inserted fragments were identified by using the colony-PCR method.
2.4. Quantitative real-time RT-PCR
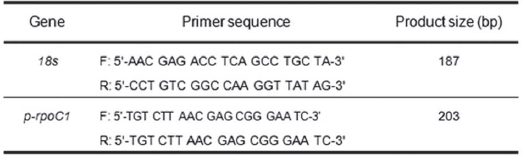
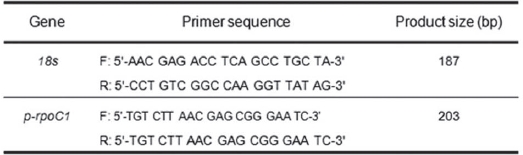
Real-time quantitative RT-PCRs detection was detected with an StepOne machine and Fast SYBR Green Master Mix (Applied Biosystem, USA) and were measured in a 96-well plate. For each well, a 20 ㎕ reaction involved 10 ㎕ of the 2 X Fast SYBR Green Master Mix, 0.5 M each of forward and reverse primer, 2.75 ㎕ of DNase-free H2O and 2 ㎕ of cDNA templates. PCR reactions were performed using the following parameters: 8 min at 95℃ and 40 cycles each of 45 s at 95℃, 45 s at 56℃ and 45 s at 72℃. PCR products were melted by gradually increasing the temperature from 60 to 95℃ in 0.5℃ steps. The identities of the amplicons and the specificity of the reaction were verified by using a melting curve analysis. Normalization of the cDNA templates was achieved by using 18S quantification. The primers presented in Table 1 were used to analyze
[Table 1] Primer for the RT-PCR
Primer for the RT-PCR
Semi-quantitative RT-PCRs were performed to compare the differential expressions of the genes in the SSH library by using gene-specific primers. Total RNA (2 μg) was used for cDNA synthesis with the First Strand cDNA Synthesis Kit (Invitrogen), and 1.0 μl of cDNAs was used as a template for the PCRs. PCR amplification was performed under the following conditions: 95℃ for 5 mins, and 30 cycles each at 95℃ for 45 s, 54℃ for 30 s, and 72℃ for 60 s. The final incubation was done at 72℃ for 5 mins. PCR products were electrophoresed in a 2% agarose gel.
2.6. Sequencing and homology analysis
PCR products were cloned in the pEC-T vector (KOMA Co., Ltd, Seoul, Korea) and were then sequenced by using ABI 3700 DNA sequencers (Perkin Space Elmer Applied Biosystems). The sequence analysis was performed using Chromas sequence analysis software. BLASTn was used to study similar nucleotide sequences.
3.1. Isolation of differentially expressed genes in wild ginseng
To identify WG-specific genes, we subtracted WG cDNAs from a pool of CG cDNAs. The subtraction was expected to significantly reduce common cDNAs and to enrich WG-specific cDNAs. More than 100 tranformants were obtained from the library, and the recombinant efficiency detected by using colony-PCR was about 90%. One hundred positive clones that had been confirmed by using PCR amplification were randomly selected, from which, 16 significantly different clones were sequenced. Because the SSH procedure includes a restricted enzyme digestion of the cDNAs produced, none of the clones obtained from the resulting libraries were full length.
Among the novel cDNAs identified here as wildginseng-specific gene was the chloroplast
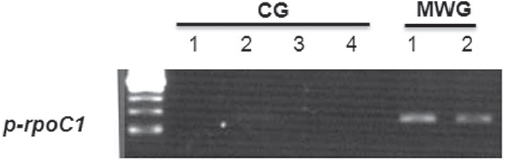
To confirm the differential expression of the
As shown in Fig. 3, all of the
3.3. Real-time RT-PCR analysis
To further verify that the
In the present study, to identify a WG-specific gene, we subtracted cDNAs expressed in WGs from those in CGs by using the SSH technique [9]. We isolated a novel gene,
The
In conclusion, although these observations suggest that the