Sophorae Radix (SR) plays a role in a number of physiologic and pharmacologic functions in many organs.
The aim of this study was to clarify the potential role for transient receptor potential melastatin 7 (TRPM7) channels in SR-inhibited growth and survival of AGS and MCF-7 cells, the most common human gastric and breast adenocarcinoma cell lines.
The AGS and the MCF-7 cells were treated with varying concentrations of SR. Analyses of the caspase-3 and - 9 activity, the mitochondrial depolarization and the poly (ADPribose) polymerase (PARP) cleavage were conducted to determine if AGS and MCF-7 cell death occured by apoptosis. TRPM7 channel blockers (Gd3+ or 2-APB) and small interfering RNA (siRNA) were used in this study to confirm the role of TRPM7 channels. Furthermore, TRPM7 channels were overexpressed in human embryonic kidney (HEK) 293 cells to identify the role of TRPM7 channels in AGS and MCF-7 cell growth and survival.
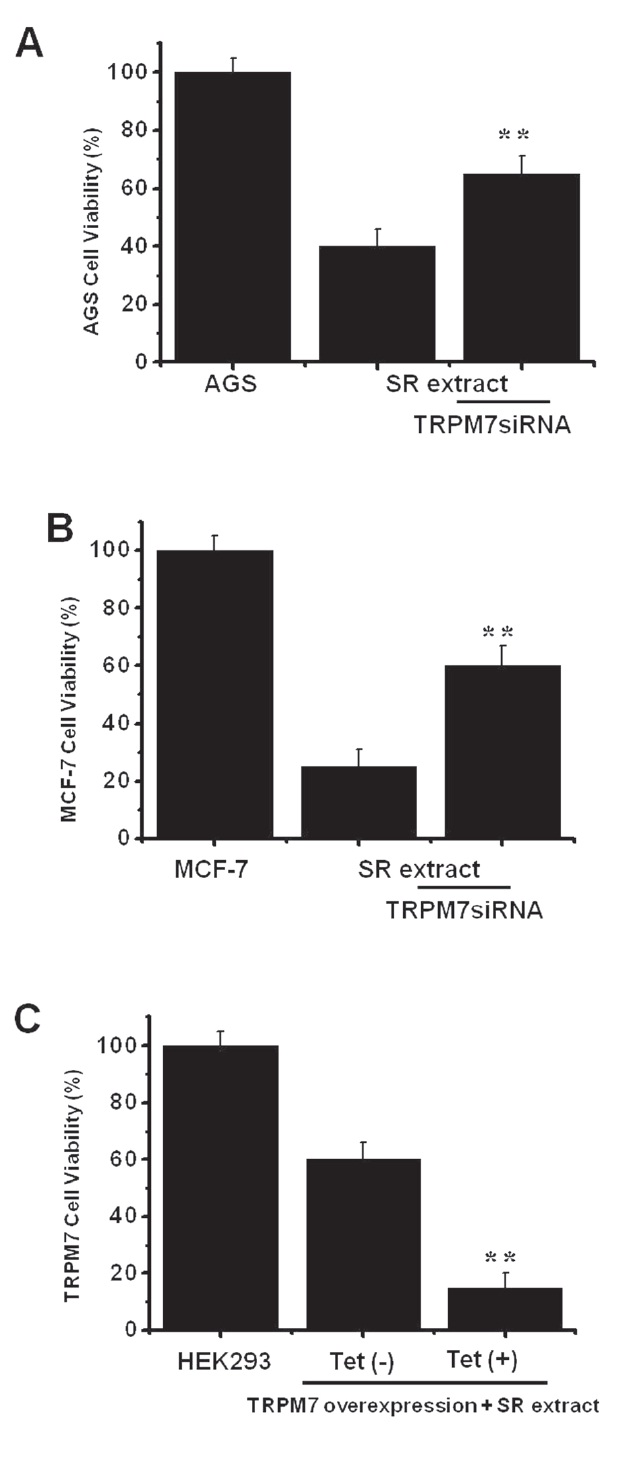
The addition of SR to a culture medium inhibited AGS and MCF-7 cell growth and survival. Experimental results showed that the caspase-3 and -9 activity, the mitochondrial depolarization, and the degree of PARP cleavage was increased. TRPM7 channel blockade, either by Gd3+ or 2-APB or by suppressing TRPM7 expression with small interfering RNA, blocked the SR-induced inhibition of cell growth and survival. Furthermore, TRPM7 channel overexpression in HEK 293 cells exacerbated SR-induced cell death.
These findings indicate that SR inhibits the growth and survival of gastric and breast cancer cells due to a blockade of the TRPM7 channel activity. Therefore, TRPM7 channels may play an important role in the survival of patients with gastric and breast cancer.
Gastric and breast cancer are leading causes of cancerrelated mortality in Korea. In previous studies, we suggested that human gastric adenocarcinoma cells expressed the transient receptor potential melastatin 7 (TRPM7) channel, which is essential for cell survival and is a potential target for pharmacological gastric cancer treatment [12]. Also Guilbert et al. suggested that TRPM7 was involved in breast cancer cell viability [13]. TRPM7 is a member of the large TRP channel superfamily that is expressed in nearly every tissue and cell type [14-16]. Many reports suggest that activated TRPM7 channels contribute to a number of physiological and pathophysiological processes [17-19]. However, the role of the TRPM7 channel in the survival of gastric and breast cancer cells after incubation with SR is unknown.
In this study, we examined the potential role of TRPM7 channels in SR-inhibited growth and survival of AGS and MCF-7 cells, the most common human gastric and breast adenocarcinoma cell lines. Our data suggest that TRPM7 channels play an important role in the survival of these tumor cells.
2.1. Preparation of SR extract
SR was purchased from Kwangmyungdang Medicinal Herbs (Ulsan, Korea). Fifty grams of SR was immersed in 1,000 ml of methanol, sonicated for 30 mins, and then extracted for 24 hrs. The extract was filtered with Whatman filter paper No. 20 and evaporated under reduced pressure by using a vacuum evaporator (Eyela, Japan). The condensed extract was then lyophilized using a freeze dryer (Labconco, Kansas City, MO, USA). Finally, 6.43 g of lyophilized powder was obtained (yield, 12.9%). The methanol extract of SR (Voucher No. MH2010-004) was deposited at the Division of Pharmacology, School of Korean Medicine, Pusan National University.
AGS and MCF-7 cell lines were used. The AGS and the MCF-7 cell lines were established at the Cancer Research Center, College of Medicine, Seoul National University, Korea. The cell lines were propagated in RPMI-1640 medium (Gibco-BRL) supplemented with 10% heat-inactivated fetal bovine serum and 20-
A whole-cell configuration of the patch-clamp technique experiment was performed at room temperature (22-25℃). The AGS or the MCF-7 cells were transferred to a small chamber on an inverted microscope stage (IX70, Olympus, Japan) and were constantly perfused with a solution containing 2.8-mmol/L KCl, 145-mmol/L NaCl, 2-mmol/L CaCl2, 10-mmol/L glucose, 1.2- mmol/L MgCl2, and 10-mmol/L 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES), adjusted to a pH of 7.4 with NaOH. The pipette solution contained 145-mmol/L Csglutamate, 8-mmol/L NaCl, 10-mmol/L Cs-2-bis(2-aminophenoxy)- ethane-
2.4. TRPM7 expression in human embryonic kidney 293 cells
Human embryonic kidney (HEK)-293 cells were transfected with the Flag-murine long transient receptor potential channel 7 (LTRPC7)/pCDNA4-TO construct and grown on glass coverslips in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, blasticidin (5
2.5. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting
Western blots were performed using the AGS or MCF-7 cell lysates. Proteins were separated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 8% polyacrylamide gels, transferred to a polyvinylidene difluoride membrane, and quantified by incubation with anti-Poly (ADP-ribose) polymerase (PARP) (1:500) antibodies. All procedures used standard methods.
All the synthetic small interfering (si) RNA sequences were designed by Qiagen with the BIOPREDsi algorithm licensed from Novartis. All siRNA target sequences that were used to silence the
2.7. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay
Cell viability was assessed by using a MTT assay. The AGS or the MCF-7 cells were seeded into each well of 12-well culture plates and then cultured in Roswell Park Memorial Institute medium (RPMI)-1640 supplemented with other reagents for 72 hrs. After incubation, 100
Caspase-3 and -9 assay kits (Cellular Activity Assay Kit Plus) were purchased from BioMol (Plymouth, PA, USA). After experimental treatment, cells were centrifuged (10000 g, 4℃, 10 mins) and washed with PBS. Cells were re-suspended in icecold cell lysis buffer and incubated on ice for 10 mins. Sample were centrifuged at 10000 g (4℃, 10 mins), and the supernatant was removed. Supernatant samples (10
2.9. Assessment of mitochondrial membrane depolarization
Mitochondrial membrane depolarization was evaluated using a JaC-1 fluorescence probe according to the manufacturer's instructions (Molecular Probes). The AGS or the MCF-7 cells were labeled with 2
Data are expressed as mean ± standard error of the mean (SEM). Differences between the data were evaluated by using the student’s
3.1. SR-extract induced cell death in AGS and MCF-7 cells
To ascertain whether SR extract kills AGS and MCF-7 cells, we performed MTT assays. The viable cell population was gradually reduced with increasing concentrations of SR extract, with a half maximal inhibitory concentration (IC50) value of 116.2
3.2. SR-extract triggered apoptosis in AGS and MCF- 7 cells
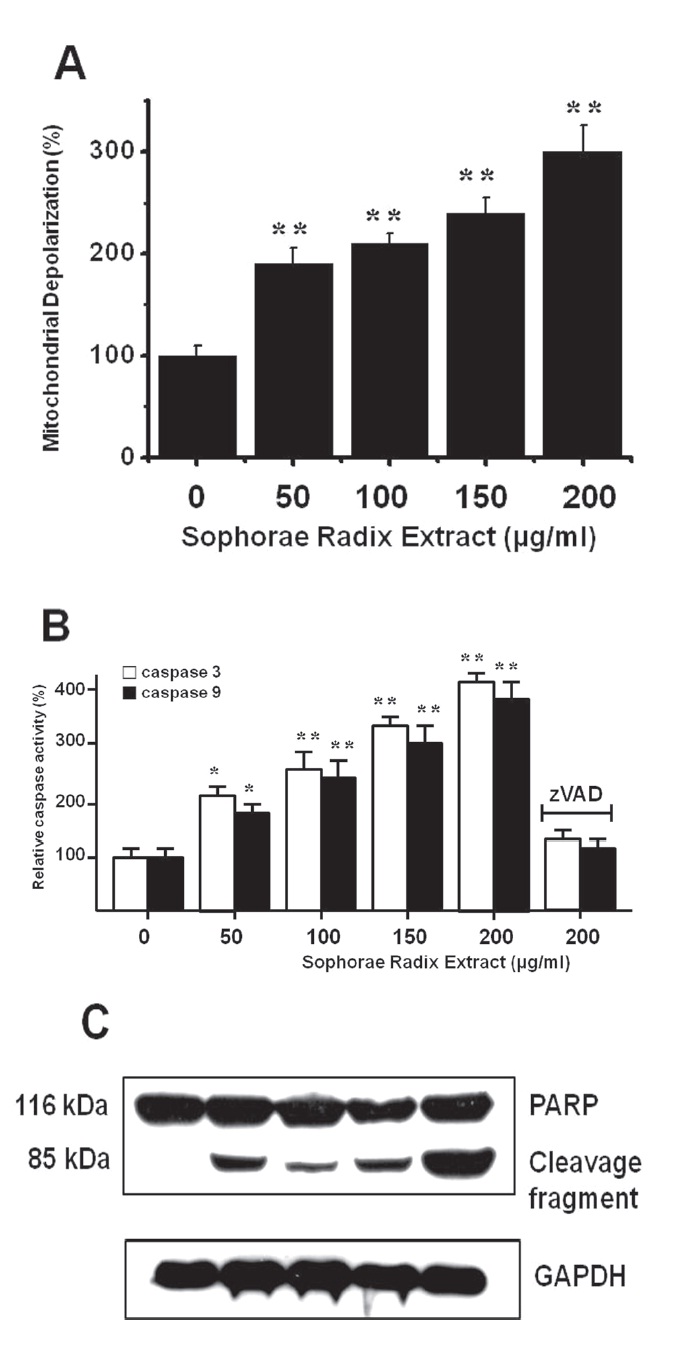
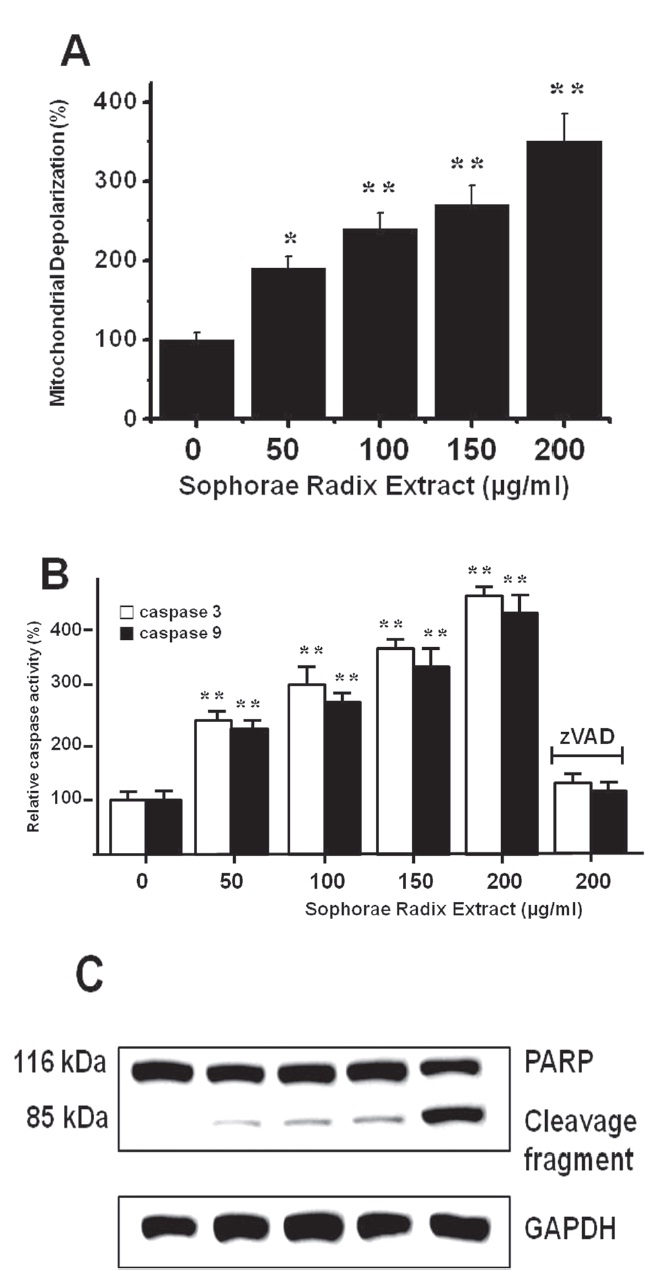
To determine whether AGS and MCF-7 cell death occurs by apoptosis, we conducted a mitochondrial membrane depolarization assay. SR extract elevated mitochondrial membrane depolarization, an early event of intrinsic apoptosis signaling (Figs. 3A and 3B). Thus, our findings suggest that SR extract induces apoptosis via intrinsic apoptotic mechanism(s). Caspase- 3 or -9 activation is one of the hallmarks of apoptotic cell death. We also measured the enzyme activity in AGS and MCF-7 cells after SR extract incubation. Using a synthetic substrate, we detected the caspase-3 and -9 activity in AGS and MCF-7 cells. SR extract increased the activities of caspase-3 and -9, which were restrained by using zVAD-fmk, a pan-caspase inhibitor (Figs. 2B and 3B). We further characterized the change in caspase-3 activity by using a western blot analysis of its natural substrate, PARP, which has been shown to function as a cellular target of caspase-3 and other caspases. During apoptosis, PARP is proteolytically cleaved from a 116-kDa-intact form into 85- and 25-kDa fragments. After incubation with SR extract for 72 hrs, AGS and MCF-7 cells showed increased PARP cleavage (Figs. 2C and 3C).
3.3. Inhibition of cell death by TRPM7 blockade
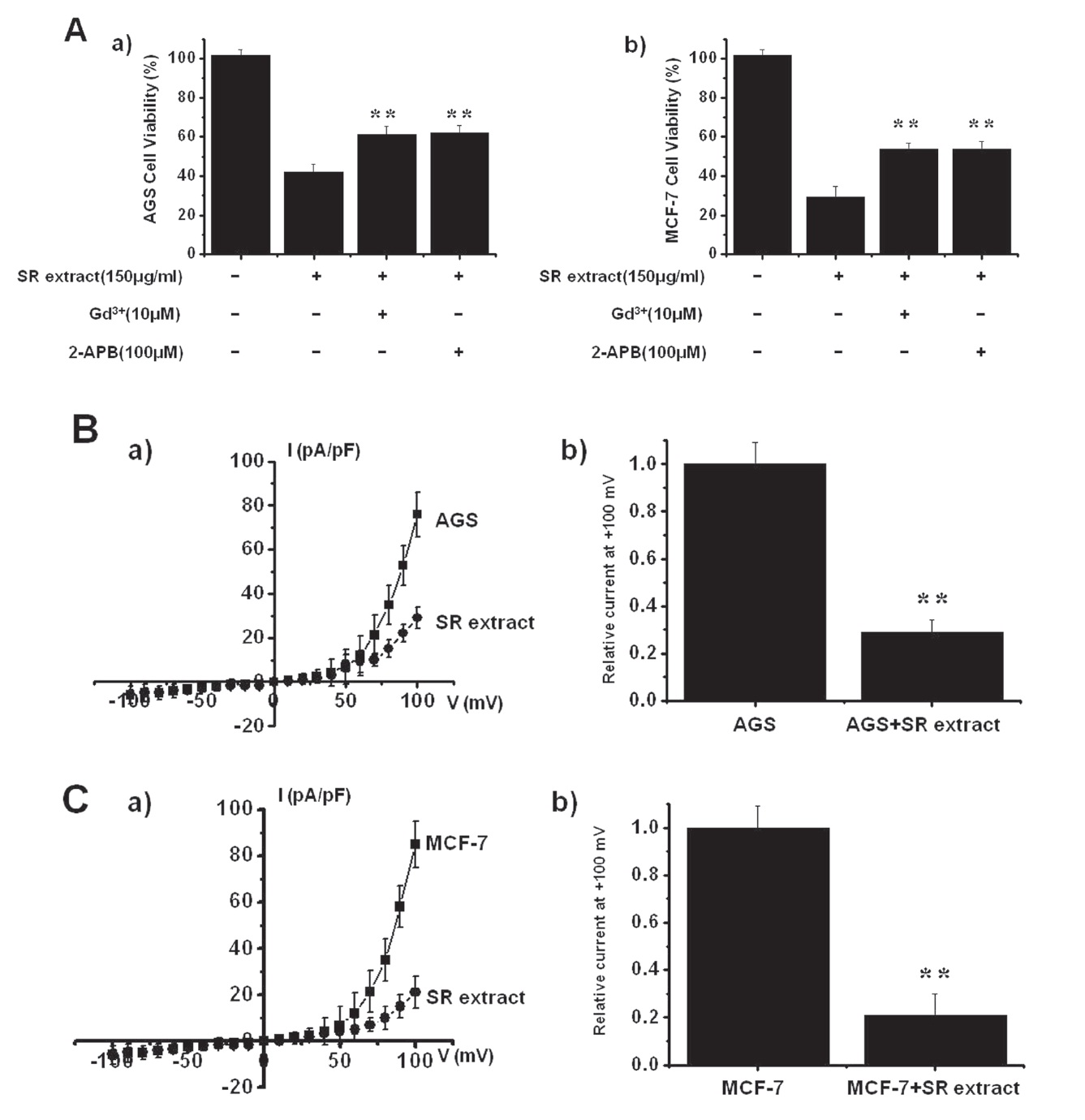
TRPM7 has been proposed to be required for cell survival on the basis of experiments on genetically-engineered DT-40 Bcells [15]. Furthermore, Guilbert et al. [13] suggested that TRPM7 was required for the proliferation of MCF-7 breast cancer cells. Also, we recently suggested, as in previous reports, that AGS cells express the TRPM7 channel and that suppression of the TRPM7 channel induced cell death [12]. Therefore, we investigated the influence of TRPM7 channels on the survival of AGS and MCF-7 cells after they had been incubated with SR extract for 72 hrs. First, we tested the effect of 2-APB, a non-specific TRPM7 channel inhibitor, on AGS and MCF-7 cell survivals. Addition of 100
MCF-7 cells (n = 6; Figs. 4Aa and b). We performed whole-cell voltage-clamp recordings to investigate the effect of SR extract on the TRPM7-like current in AGS and MCF-7 cells. A voltage ramp with a voltage ranging from +100 mV to -100 mV evoked small inward currents at negative potentials whereas larger outward currents, showing outward-rectifying cation currents, were evoked at positive potentials (n = 5; Figs. 4B and C). In the presence of SR extract, the amplitudes of the currents were inhibited outwardly by 29.3 ± 5.1% and inwardly by 3.1 ± 2.3% in AGS cells and outwardly by 21.4 ± 4.2% and inwardly by 4.1 ± 3.1% in MCF-7 cells (n = 10; Figs. 4B and C).
3.4. Effects of RNAi on AGS and MCF-7 cells
We used RNA interference (RNAi) to determine if the TRPM7 channel was actually important to cell viability after incubation with SR extract. To prevent non-specific effects of the siRNA sequence used here, we generated 3 types of 21-nucleotide siRNA that targeted human TRPM7 specifically, TRPM7siRNA1, TRPM7siRNA2, and TRPM7siRNA3 [12]. In our previous report, we studied the effects of these TRPM7siRNA sequences on AGS cells. Only the TRPM7siRNA3 sequence decreased TRPM7 protein expression by 70-80% without reducing glyceraldhyde- 3-phosphate dehydrogenase (GAPDH) expression [12]. We used the TRPM7siRNA3 sequence in this experiment. After cell incubation with SR extract and transfection with TRPM7siRNA3, viability increased from 40.2 ± 2.3% to 65.4 ± 3.1% in AGS cells and from 25.5 ± 3.2% to 60.2 ± 5.3% in MCF-7 cells in the MTT assays (
Plasma membrane ion channels participate in cellular electrogenesis, electrical excitability, virtually all basic cellular behaviors, including such crucial ones for maintaining tissue homeostasis as proliferation, differentiation, and apoptosis [20-23]. The involvement of ion channels in the regulation of cellular proliferation or apoptosis in vitro has been known since, at least, the late 1980s based on observations that classical blockers of these channels can influence the rate of cell death by prolonging or shortening cell survival. Ion channels are crucial to tumor development and cancer growth. Epithelial cells change from normal to cancerous while a series of genetic alterations occur, which may also affect ion channel expression or cause changes in ion channel activity [24]. Voltage-gated K+ channels are overexpressed in colon cancer [25], and voltagegated sodiumion channels are involved in the growth of prostate cancer [26]. Volume-regulated Cl- channels have been observed in a human prostate cancer cell line and in lung cancer cells [27-29].
The TRP superfamily of channels combines recently-identified ubiquitously-expressed channels sharing a high degree of structural homology. All identified TRP channels are tetramers composed of identical or closely homologous subunits belonging to the same subfamily [30-32]. At present, the mammalian TRP superfamily consists of 28 members grouped into smaller subfamilies by homology, typically having similar functional properties as well: TRPC (canonical), TRPV (valinoid), TRPM (melastatin), TRPML (mucolipin), TRPA (ankyrin-like) and TRPP (polycystin). TRP proteins have diverse functional properties and have profound effects on a number of physiological and pathological conditions. The majority of the evidence of TRPV channels affecting cell development and survival, especially in connection with cancer, is focused on the TRPV6 ion channel. TRPV6 is involved in prostate adenocarcinomas and is a promising target for new therapeutic strategies for treating advanced prostate cancer [29,33]. TRPV1 has at least a casual role in regulating the cell cycle. Expression levels of TRPV1 are known to be increased in prostate, colon, bladder and pancreas cancers [29,32,34-37]. TRPM1 appears to be a prognostic marker for melanoma metastasis in human cutaneous melanoma [29,38]. In addition, the TRPM8 channel protein has been used as a prostate-specific marker; the loss of TRPM8 is considered a sign of poor prognosis [29,39,40]. TRPM7 is endogenously expressed in a wide variety of tissues, including brain, hematopoietic [41], kidney, and heart [42,43] tissues. The TRPM7 cation channel supports multiple cellular and physiological functions, including cellular Mg2+ homeostasis [44], cell viability and growth [15,45], anoxic neuronal cell death [46], synaptic transmission [47], cell adhesion [48], and intestinal pacemaking [29,49]. Wykes et al. [50] suggested that TRPM7 channels were critical to human mast cell survival. Jiang et al. [18] suggested that activation of TRPM7 channels was involved in the growth and proliferation of human head and neck carcinoma cells. Abed et al. [51] proposed the importance of TRPM7 in the human osteoblast-like cell proliferation. As in previous studies, Guilbert et al. [13] suggested that TRPM7 was required for breast cancer cell proliferation. Also, we suggested that TRPM7 channels played an important role in the growth and the survival of gastric cancer cells [12]. In line with these studies, our studies show that SR induces apoptosis in human gastric and breast adenocarcinoma cells, which may be due to a blocking of the TRPM7 channel activity.
SR has been used as a constituent of oriental herb medicine prescriptions to produce the variety of physiologic or pharmacologic effects in various regions. However, only a few reports have described the effects of SR on gastric and breast cancer. In a previous report, evidence indicated that TRPM7 channel activation influenced the growth and the survival of human gastric and breast adenocarcinoma cells [12,13].
In conclusion, SR inhibited the growth and the survival of AGS and MCF-7 cells. Caspase-3 and -9 activity were elevated, and the degree of PARP cleavage was increased. Blockade of TRPM7 channels by Gd3+ or 2-APB or suppression of TRPM7 expression by siRNA blocks SR induced inhibition of cell growth and survival. Furthermore, overexpression of TRPM7 channels in HEK 293 cells increases the rate of SR-induced cell death. These findings indicate that SR inhibits the growth and survival of gastric and breast cancer and that the SR-induced apoptosis is due to the blockade of TRPM7 channel activity. Therefore, TRPM7 channels may play an important role in survival in cases of gastric and breast cancer.

![Sophorae Radix (SR) induces cell death in AGS and MCF-7 cells. (A) The AGS cells were incubated with SR at the indicated concentrations for 72 hrs prior to MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assays. Distilled water was used as a vehicle. Cell viability is expressed as a value relative to that of the untreated cells which is set to 100%. (B) Time course response to SR. AGS cell viability is expressed as a value relative to that of the cells treated with a vehicle and harvested at zero time which is set to 100%. (C) The MCF-7 cells were incub-ated with SR at the indicated concentrations for 72 hrs prior to MTT assays. Distilled water was used as a vehicle. Cell viability is expressed as a value relative to that of the untr-eated cells which is set to 100%. (D) Time course response to SR. MCF-7 cell viability is expressed as a value relative to that of the cells treated with a vehicle and harvested at zero time which is set to 100%. The figures show mean ± SEM. *P? 0.05, **P? 0.01.](http://oak.go.kr/repository/journal/10929/DHOCBS_2012_v15n3_31_f001.jpg)