Hydrogenated amorphous carbon (a-C:H) films have been widely investigated owing to their numerous potential applications in hard coatings, lithography, electroluminescence (EL)devices, field emission devices[1-4]. a-C:H’s emitting property has attracted considerable interest due to potential uses in EL devices [5,6]. Understanding thermal stability is important for the practical application of EL devices, since emission of a-C:H thin film accompanies heat generation inside the film. Photoluminescence(PL) of a-C:H thin film is known to be quantum confinement effect of localized carriers in
To the best of our knowledge, the observation of microstructural change during emission has seldom been reported in a-C:H thin film. Only a couple of publications have reported the annealing effect for a-C:H thin film.
In this letter, we report the emitting properties of a-C:H thin film during spontaneous emission using a He-Cd laser source.Additional thermal annealing was also performed to determine what kind of degradation occurred during the spontaneous emission. Detailed characterizations of the microstructure are performed to explain the relationship between the microstructure and the emission property.
[Table 1.] FT-IR transmission spectrum of a-C:H films.
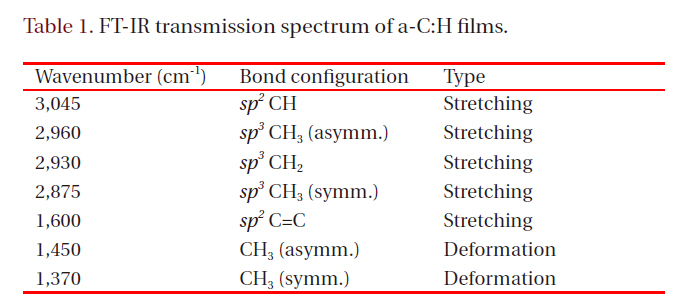
FT-IR transmission spectrum of a-C:H films.
Commercial
The 325 nm line of He-Cd laser (double spectrometer; SPEX 1403, USA) was used as a photoexcitation source. The change of PL spectra was taken during excitation. a-C:H thin films were annealed from 100℃ to 400℃ in atmospheres to determine the thermal effect during the photoexcitation. Microstructures of the films were analyzed using Fourier transform infrared (FT-IR)spectroscopy (spectrum 2000; Perkin Elmer, UK). IR transmission spectra, ranging from 400 to 4,000 cm-1, were taken in a N2 atmosphere to minimize H2O vapor influence.
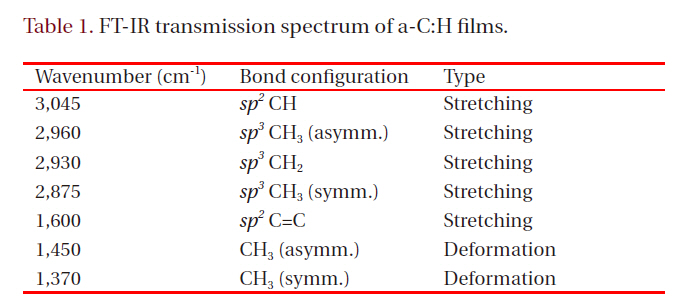
a-C:H thin films were irradiated to a typical He-Cd laser to study emitting stability. Figure 1 shows the dependence of PL intensity on the laser irradiation time. As seen in Fig. 1, the PL intensity gradually increased by 170% with the irradiation time up to 2,000 seconds. After reaching the maximum intensity at
around 2,000 seconds, the PL intensity started decreasing very slowly. The PL intensity was still 1.7 times higher than the initial value, even 1,000 seconds after the maximum. The inset in Fig.1 shows PL spectra for the a-C:H thin film at 0 second and 2,000 seconds that correspond to ‘A’ and ‘B’ in Fig. 1. As seen in the inset,Full width half maximum of the PL peak increased from 0.45 eV to 0.58 eV with the laser irradiation time. The center of the PL peak shifted to the lower energy to 2.28 eV after laser irradiation of 2,000 seconds.
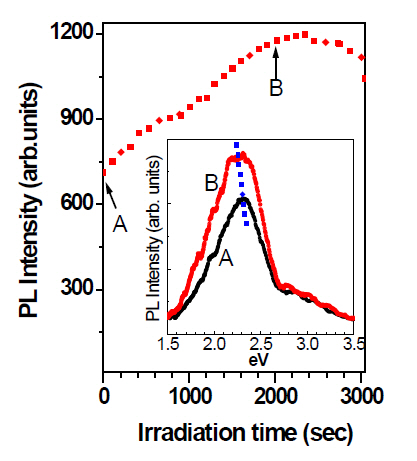
The FT-IR method was exploited to investigate structural change of a-C:H thin film during the laser irradiation, as shown in Fig. 1. Figure 2 shows FT-IR spectra of a-C:H thin film before and after laser irradiation, at elapsed times of 0 second and at 3,000 seconds, respectively. Table 1 identifies each absorption band. The IR absorption band area between 2,700 cm-1 and 3,200 cm-1 represents the relative amount of bonded hydrogen in a-C:H film [9]. Considering this, well separated peaks of as-deposited a-C:H thin film between 2,700 cm-1 and 3,200 cm-1 advise the presence of C-H bonds in a polymer-like structure with high hydrogen content. In contrast, the a-C:H thin film deposited after laser irradiation contains fewer C-H bonds than those of the as-deposited film in terms of a drastic decrease of the absorption area in the range. This means a-C:H thin film suffers transformation from a polymer-like to a graphite-like phase under laser irradiation.
The remaining energy is expected to be converted into heat,since only a small portion of energy from laser energy irradiating a-C:H thin film can be used to excite the radiative recombination of the electron and hole pair. In turn, the generated heat could preferentially break the weaker C-H bond (3.5 eV) than the C-C bond (25 eV), resulting in dehydrogenation. In this respect, it can be seen that the dehydrogenation by laser irradiation up to 2,000 seconds increases sizes of the
As deposited a-C:H thin films were annealed at temperatures ranging from 100℃ to 400℃ to investigate thermal effects of laser irradiation on C-H bonding in a-C:H film.
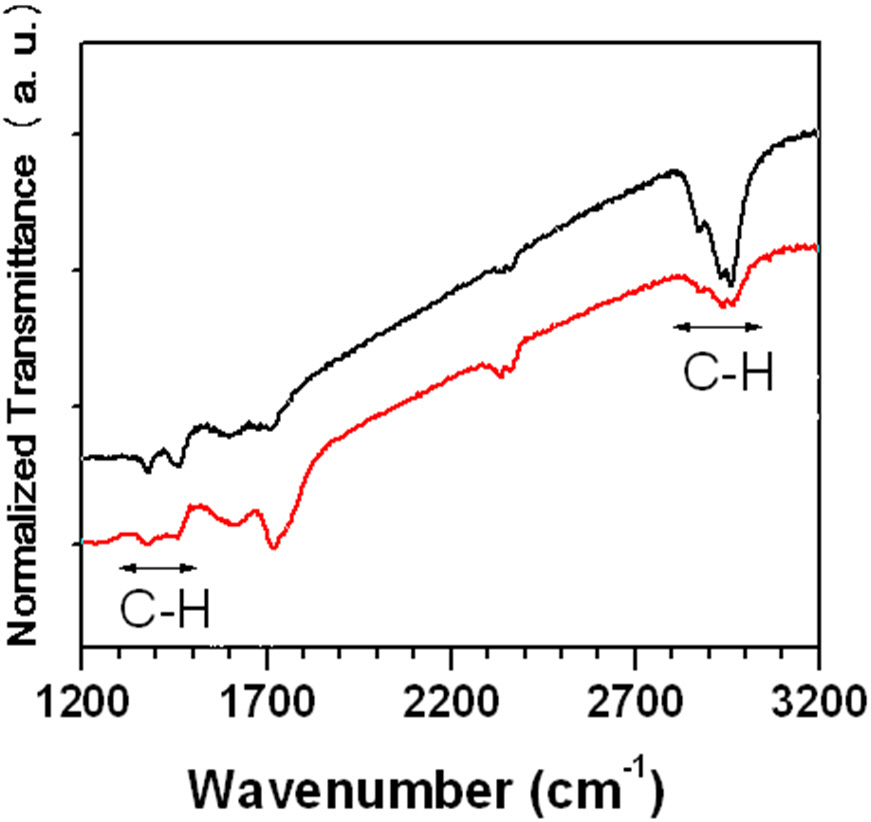
Figure 3 shows IR spectra of as-deposited and annealed a-C:H thin films at different temperatures. Well-separated peaks of asdeposited a-C:H thin film between 2,700 cm-1 and 3,200 cm-1 lasted up to annealing temperature of 200℃. The area of the absorption band between 2,700 cm-1 and 3,200 cm-1 showed only a slight decrease, as the annealing temperature (T
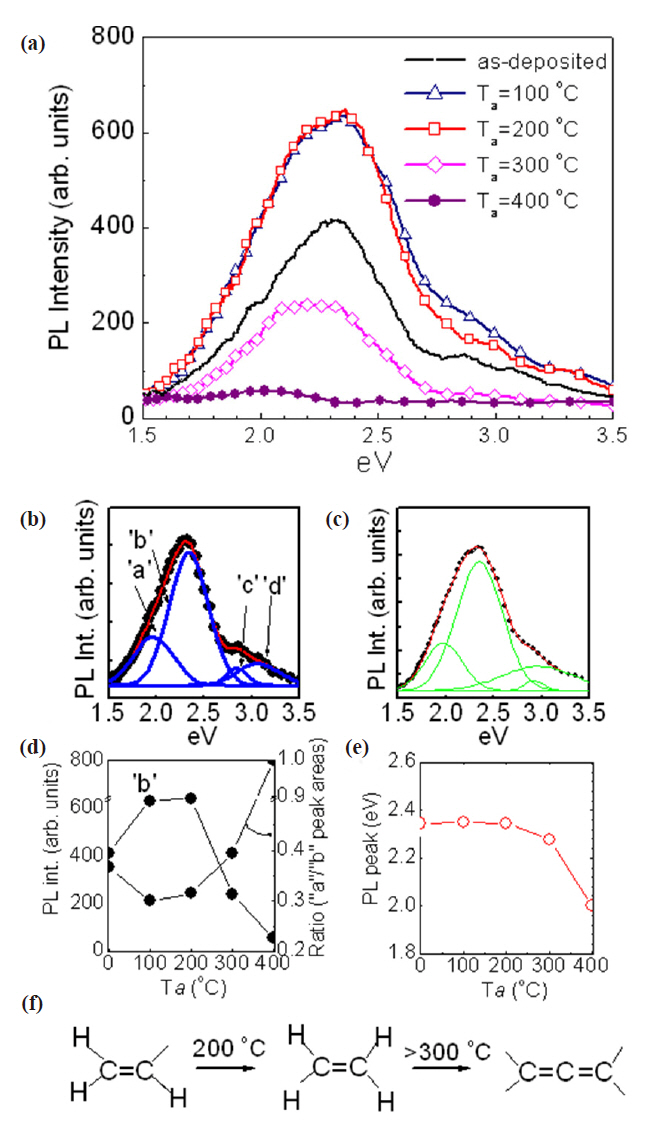
Figure 4 shows dependence of PL on various annealing temperatures for a-C:H thin films. PL spectra were deconvoluted into four individual peaks, indicating the size of C=C cluster had various PL in a-C:H thin film. Figures 4(b) and (c) are examples of the deconvolution for as-deposited a-C:H thin films and annealed at 100℃, respectively.
As the annealing temperature increased up to 200℃, PL intensity drastically increased and reached a maximum. After reaching the maximum, the PL intensity considerably decreased, as the annealing temperature increased from 200℃ to 400℃.
Figure 4(d) showed intensity changes ‘b’ and ‘a’ peak and its ratio in (a) as a function of T
The significant decrease of ‘b’ peak intensity from 200℃ to 300℃ in Figs. 4(a) and (d) corresponds to considerable reduction of the FT-IR absorption band between 2,700 to 3,200 cm-1 in Fig.3. Thus, excessive dehydrogenation occurs in the temperature range from 200℃ to 300℃. Increase of
a-C:H film was deposited on a Si(100) substrate by DC saddle field PECVD. A He-Cd laser was used to induce PL of a-C:H. The PL intensity achieved a maximum value that increased to 170%with the irradiation time up to 2,000 seconds. a-C:H thin film was in a polymer-like phase before laser irradiation, but suffered transformation to graphite-like phases after laser irradiation.
Although some energies of He-Cd laser are used to excite the electron-hole pair, the remainder generates heat, resulting in structural changes in the a-C:H film. The thermal energy positively contributed to the increase of PL intensity at the initial stage, and then had a negative effect in the next stage, leading to a decrease of PL, together with a red shift of the PL peak.
The annealing of the a-C:H thin film reveals non-radiative defects were reduced by the heat generation in the initial stage,but excessive heat generation causes dehydrogenation in the next stage, leading to an increase of non-radiative defects again.That is, thermal energy reduced non-radiative defects, mainly dangling bonds, probably by connecting them to non-bonded hydrogen at the initial stage. However, excessive thermal energy initiates dehydrogenation, which increases non-radiative defects and