There are numerous studies on the synthesis of semiconductor nanocrystals (NCs) (quantum dot) due to their importance in optoelectronic, photovoltaic and biological applications [1]. Since the synthesis of monodispersed CdSe NCs by hot injection using organometallic precursors was reported [2], most experimental work has been devoted to II-VI semiconductors, such as CdSe, CdS, and CdTe, due to their high quantum yield (QY) of fluorescence with tunable emission wavelength. However, heavy metals, such as Cd, Pb, and Hg, in the II-VI semiconductor NCs have limited applications due to their intrinsic toxicity. As alternatives, ternary chalcopyrite-type I-III-VI2 group semiconductors with less-toxic components have been proposed [3-7]. Among these, CuInS2 (CIS) with a bulk band gap of 1.5 eV was selected as an important candidate for optical application. Work on the synthesis of CIS NCs and efficient route to highly luminescent CuInS2/ZnS core shell NCs has been reported [5-9]. Zhong et al. [4] reported synthesis of CIS NCs using copper acetate, indium acetate and 1-dodecanethiol by thermolysis of intermediate, CuIn(SR)x compound. Uehara et al. [10] and Nakamura et al. [11] improved the photoluminescence (PL) of CIS NCs by introducing lattice defects through highly off-stoichiometric composition from CuInS2. Further, surface capping with the larger band gap compounds improved PL by reducing surface states and charge confinement. The present authors reported highly improved luminescence QY up to 71% by refluxing the NCs with zinc acetate and palmitic acid [12].
Park and Kim [9] reported a novel shell formation route for CIS NCs by adding zinc salts powders. The large improvement in fluorescence intensity was attributed to the formation of ZnS shell layers by cation exchange reaction. In addition, the emission wavelength showed a significant blueshift after formation of ZnS. Park and Kim [9] attributed this to the size reduction of CIS cores after cation exchange reaction. Li et al. [8] also assessed that the blue shift is indicative of etching of core materials and the increased degree of spatial confinement. However, Uehara et al. [10] described the origin of this blueshift with partial alloying of CIS NCs with ZnS.
In this study, we synthesized Cu-deficient CIS NCs with Zn1-xCdxS alloyed shell layers of various compositions. We controlled the band alignment of NCs by alloyed shell layers and analyzed their luminescence with a steady state and time-resolved PL (TRPL). In addition, the origin of long lifetime in the I-III-VI semiconductor NCs was elucidated.
CIS NCs were synthesized using copper acetate, indium acetate and 1-dodecanethiol (DDT), following the protocol reported by Zhong et al. [6]. The amount of Cu (II) acetate was varied from 0.2 mmol to 1 mmol and 1 mmol of indium acetate was used. Further minimization of Cu acetate retarded the formation of NCs. Those precursors were dispersed in 30 mL of 1-octadecene under an inert atmosphere at room temperature and subsequently heated to 240℃. After reaction for 15 minutes, the solution was cooled to room temperature. One mmol of zinc acetate was added to the CuInS2-based core solution after cooling to room temperature without purification and separation of synthesized NCs to construct core-shell structure. Three mmol of palmitic acid mixed with 10 mL of 1-octadecene was also added to the solution for colloidal stability of NCs. Subsequently, the mixture was heated to 230℃ under an inert N2 atmosphere and reacted without further addition of precursors. The mixed solution became uniform above 120℃ and further dwelling at 230℃ to enhance the luminescence. After refluxing for 5 hours, DDT with quantity equivalent to zinc acetate was injected to the solution and maintained for an hour to form the ZnS shell at the same temperature. Aliquots were taken at the end of each step and rapidly cooled to room temperature. A polar solvent, such as acetone, or methyl alcohol, was added to precipitate CuInS2-based NCs before further characterization. The precipitates were purified by repetition of centrifugation and re-dispersion to nhexane.
The luminescence QY was calculated from comparison to the luminescence intensity of organic dye, such as Rhodamine 6G dissolved in ethanol (QY = 0.95). Samples for temperature dependent PL and Raman spectroscopy were prepared by drop casting the nanocrystal (NC) solution on the Si substrate. Powder X-ray diffraction (XRD) profiles were obtained with Rigaku D-1200 (Rigaku, Tokyo, Japan). The absorption spectra were obtained with a UV-VIS spectrometer (Cary 5000; Varian, Palo Alto, CA, USA) and PL data were measured by a fluorophotometer (FP-550; JASCO, Tokyo, Japan). The PL dynamics were studied with TRPL excited at 375 nm by 50 ps pulses at a 500 kHz repetition rate and detected with a time-correlated single photon counting system.
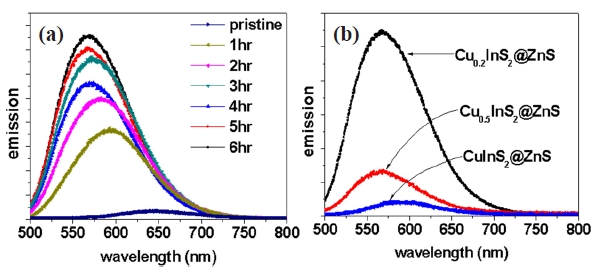
Refluxing the Cu-deficient CIS NCs with zinc acetate and palmitic acid showed variation in emission wavelength and a clear enhancement of emission intensity, as shown in Fig.1 (a) [12]. This improvement in PL intensity was attributed to the formation of ZnS shell layers on Cu-deficient CIS cores. The blueshift in the emission wavelength was as large as ~80 nm. The blueshift in the emission wavelength after the ZnS shell layer formation was reported by several different groups [5-11]. Although several explanations on the blueshift were proposed, the origin of this blueshift is still under debate, as mentioned earlier. In this study, we explored the origin of the blueshift in PL and intended to manipulate the emission wavelength by alloying the shell layers.
Various reports have shown that the main fluorescence process in CIS NCs is closely related to lattice defects, such as copper vacancy, and indium substitute anti-site defect [6,8]. Then,
intentional introduction of copper vacancies by the highly nonstoichiometric composition improved the PL of CIS NCs, as shown in Fig.1 (b). In addition, the emission wavelength of ZnScapped CIS NCs was blueshifted with a large copper deficiency by ~30 nm. This blueshift was explained by widening of band gap due to weakened contribution of copper d electrons in the valence band due to the copper vacancy as reported for Cu(In,Al)S2 [13]. The contribution of copper deficiency to the blueshift in PL of ZnS-capped CIS NCs was much smaller compared to the blueshift after ZnS capping of CIS NCs. In addition, the large blueshift after ZnS capping was observed in Cu0.2InS2@ZnS NCs having a large copper deficiency, irrespective of surface capping. Then, the contribution of the copper vacancy to the blueshift in PL of ZnS-capped CIS NCs was quite negligible.
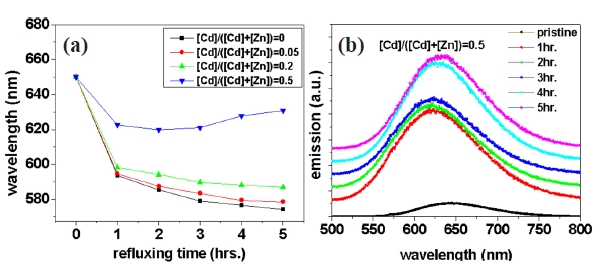
Figure 2(a) shows the change of emission wavelength with respect to reaction time during the growth of shell layers with various Cd/Zn ratios. We successfully minimized the blueshift in PL after shell layer formation by increasing the portion of cadmium in the shell layers. In particular, CIS NCs with shell layers of Cd:Zn = 1:1 exhibited very small blueshift, as shown in Fig.2 (b). The blueshift after capping by alloying of Zn or Cd with CuInS2 is not plausible, since both ZnS and CdS have much larger band gap energy compared with CuInS2. The possibility of the contribution of surface etching during the shell capping process can be eliminated by considering the reduced blueshift in the CIS NCs with Cd-containing shell layers.
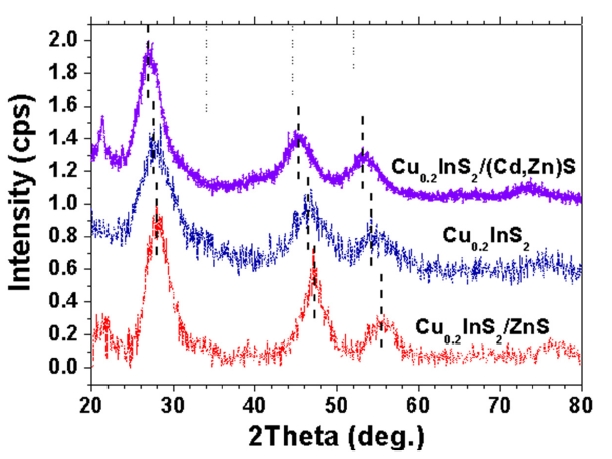
The powder XRD profiles of CIS NCs with shell layer formation presented in Fig. 3 showed a peak shift with respect to that of bare CIS NCs. The XRD peak shift in core-shell NCs after shell capping was evidence of shell formation [9,12]. In particular, Cu0.2InS2@(Cd,Zn)S NCs showed a shift to a smaller diffraction angle, in contrast to Cu0.2InS2@ZnS NCs showing a shift to a higher diffraction angle in the XRD profile. The difference in lattice parameters between core and shell materials can explain these shifts. XRD peaks were shifted to opposite direction after shell capping, since the lattice parameter of ZnS is smaller than
CuInS2 and that of CdS is larger than CuInS2 (Cu0.2InS2: a = 0.5517 nm, ZnS: a = 0.5345 nm, CdS: a = 0.582 nm). One interesting thing is the lattice strain applied by shell formation. It is known that band gap of NCs can be shifted by the lattice stress originated from the lattice mismatch between core and shell layers [14]. It is quite natural to expect shift to a smaller wavelength or blueshift in PL spectra after ZnS capping on CIS NCs, since ZnS with a smaller lattice parameter than CuInS2 can give rise to a compressive stress on CIS cores and the band gap deformation potential (dEg/dlnV) is negative. In contrast to this, the formation of the alloyed capping layer, (Cd,Zn)S shifted the XRD profile to a lower angle, indicating the tensile stress on CIS cores by lattice mismatch between core and shell layers. Then, the development of tensile stress in CIS cores resulted in redshift or minimization of blueshift in PL after shell formation. Hence, it was concluded that the lattice strain applied by lattice mismatch between core and shell layers resulted in blueshift of PL spectra by shell formation. In addition, the blueshift in PL after surface capping can be minimized by increasing the lattice parameters of the shell layers by alloying.
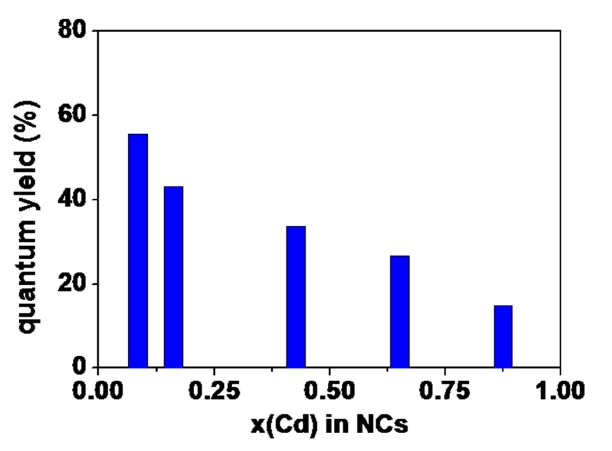
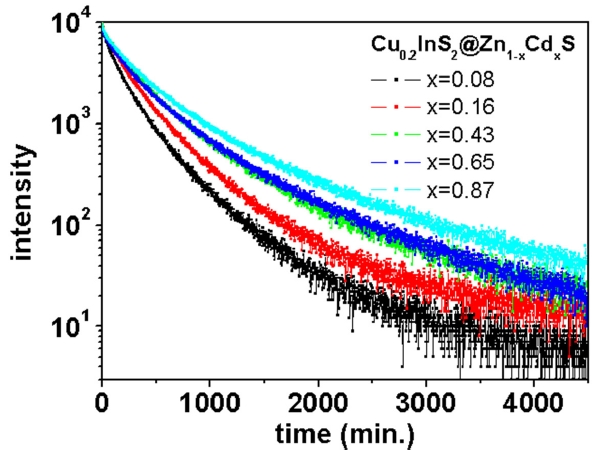
One interesting feature in CIS NCs with (Cd,Zn)S shell layers is a monotonous decrease in QY by increasing the portion of cadmium in the shell layers, as shown in Fig. .4 Furthermore, the decay of PL after pulsed excitation was highly mitigated by the increasing portion of cadmium in the shell layers, as shown in Fig. .5 The average life time (τave) was extracted from data fitting with the multi-exponential decay model:
The average life time was changed from 180 ns for the lowest cadmium content through 340 ns for the highest cadmium content. That is, QY monotonously decreased and the relaxation time was nearly doubled by increasing the amount of cadmium in the shell layers. It is known that the steady-state and the transient PL of core/shell semiconductor NCs were highly dependent on the band alignment [8,15,16].
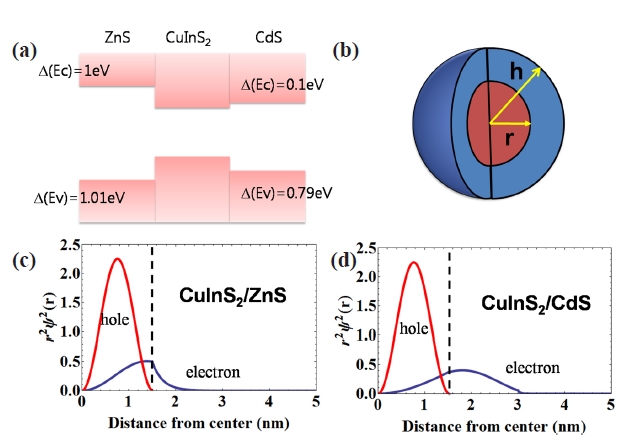
The band alignment of the bulk heterojunction shown in Fig.6 showed that the potential barrier at the conduction band of CuInS2/ZnS junction was highly minimized at the CuInS2/CdS junction. The calculation of the electron/hole wave function based on spherical potential shown in Fig.6 (b) showed a different probability distribution due of these potential barrier differences. The radial probability distribution of electrons in CuInS2/CdS core/shell NCs was highly delocalized to core and shell, in contrast to CuInS2/ZnS core/shell NCs showing highly confined electron wave function.
The wave function overlap between electron and hole was minimized, which reduced the PL intensity of NCs, since the electron wave function of Cu0.2InS2/CdS was highly delocalized.In addition, the small wave function overlap provided lower chance of electron-hole recombination and increased the lifetime of the excited electron-hole. Thus, the band alignment of NCs can be changed by shell composition and the increasing portion of Cd in shell layers for CIS NCs can reduce QY and increase the carrier life time due to reduced electron confinement.
The large blueshift in ZnS-capped CuInS2 NCs was minimized by alloying the shell layers. NCs with alloyed cores were synthesized by refluxing the as-synthesized CuInS2 NCs with a mixture of cadmium acetate, zinc acetate and palmitic acid. The PL spectra showed clear red-shift with significant minimization of emission intensity. A detailed study on the emission process of NCs implies that the formation of shell layers with small lattice mismatch minimized the mismatch strain generated from the shell layers. In particular, the TRPL spectra of the NCs showed a significant increase in the lifetime of excited carriers. The band alignment of NCs can be changed by shell composition and the increasing portion of Cd in shell layers for CuInS2 NCs can reduce QY and increase the carrier life time due to reduced electron confinement.