Organic pollution causes eutrophication and dystrophication, which occur when excessive amounts of organic matter enters seawater. Eutrophication can contaminate sediment and harm aquaculture. Polychaeta species have been shown to restore eutrophic sediment. In this study, we used peptone to simulate a eutrophic environment and detect the levels at which eutrophication became toxic to the polychaete Perinereis aibuhitensis. Peptone concentrations were 0, 100, 200, and 500 mg/L. The median lethal concentrations were 950.35 mg/L at 48 h, 340.34 mg/L at 72 h, and 120.22 mg/L at 96 h, which are much higher than those of other aquatic species. Polychaeta species are highly tolerant of eutrophication. During the 15-day long-term experiment, sediment loss on ignition, as well as seawater total organic carbon and total nitrogen all decreased significantly (P<0.05). However, NH4+ concentration increased with time. Perinereis aibuhitensis slowed the increment of NH4+ but could not prevent its increase. Our results indicate that this polychaete is helpful in the recovery of seawater and sediment from eutrophication.
The human population has increased drastically during the last several decades, with industry and agriculture developing rapidly. Environmental problems caused by rapid development are now coming to the forefront. In coastal areas, substantial waste is released into the marine environment. These anthropogenic inputs can cause eutrophication of seawater and sediment (Lenhart et al., 2010). Nutrient enrichment due to anthropogenic activities appears to be the main cause of eutrophication in coastal areas (Cloern, 2001). Seawater eutrophication can increase biomass production (Cadee and Hegeman, 2002) and lead to changes in species composition (Philippart et al., 2000). In combination with adverse meteorological and hydrographical conditions, eutrophication is an important factor in oxygen depletion and subsequent mortality of benthic communities (Meyer-Reil and Koster, 2000).
Peptone, an enzyme that digests animal protein, is commonly used as a source of organic nitrogen in microbiological and cell culture media, as well as for preparing eutrophic seawater (Matsushige et al., 1990).
The polychaete
However, relatively few studies have investigated environmental recovery in eutrophic areas. We evaluated the toxicity of peptone-synthesized eutrophic seawater to
>
Animal and sediment collection
The polychaete
>
Acute toxicity and degradation experiment
Peptone (Sigma-Aldrich, St. Louis, MO, USA) dissolved in seawater was used as an organic pollutant to create synthetic eutrophic seawater. The primary tests for acute toxicity were conducted using concentrations of 100 and 1,000 mg/L for 1 day. None of the animals in the 100-mg/L group died, whereas all of those in the 1,000-mg/L group died. Based on these results, we prepared a series of peptone test concentrations of 0, 100, 200, and 500 mg/L for the formal acute toxicity bioassays. In the long-term experiment, a concentration of 100 mg/L resulted in no deaths over 48 h.
For the experiment, 30 polychaetes (body length 16.0±1.3 mm; body weight 0.4±0.1 g) were placed in a 10 L tank with 5 cm sediment on the bottom. The volume of circulating seawater was 30 L. Average water quality conditions during the experiment were 22℃ , pH 7.5, and salinity 30.6 practical salinity unit. Air pumps and air stones provided water and sediment oxygenation and turbulence. Each experimental group was replicated three times. During the acute toxicity bioassays, the polychaetes were examined every 24 h, and dead animals were removed. For the 15-day long-term experiment, seawater and sediment samples were collected at 0, 2, 5, 7, 9, and 15 days and stored at -20℃ for further analysis.
Sediment subsamples were first analyzed to determine the LOI concentration at 0, 2, 5, 7, and 15 days, according to the Korean standard method for sediments and seawater. Sediment samples were dried and ground to a fine powder smaller than 230 mesh using a mortar and pestle. A known weight of sample was placed in an aluminum crucible, which was then ignited in a muffler furnace at 550℃ for 2 h. The sample was cooled in a dessicator and weighed. LOI was calculated as the difference between the initial and final sample weights, divided by the initial sample weight times 100%.
TOC and TN of seawater samples were analyzed at 0, 2, 5, 7, 9 and 15 days using a TOC/TN analyzer (TOC-V/TNM-1; Shimazu, Kyoto, Japan).
Seawater NH4+ was detected using the o-phenylphenol [(1, 1´-biphenyl)-2-ol] (OPP) method of Yamaguchi and Machida (1968), as modified by Kanda (1995). The method is based on the reaction of indophenols with o-phenylphenol [(1, 1´-biphenyl)-2-ol] (the Berthelot reaction) as an alternative to phenol. The extracted indophenol was used to recover ammonium nitrogen to determine the 15-N isotope ratio and, thus, the concentration of NH4+ in seawater using a Europa Scientific ANCA-MS system (Europa Scientific Ltd., Crewe, UK).
Statistical comparisons of mean cumulative mortalities were examined using an ANOVA test at α=0.05. Lethal concentrations of peptone, defined as those at which the test organisms suffered 50%mortality (LC50), and their 95%confidence limits were estimated at 24 h and 48 h using a Probit analysis (Finney, 1971). The safe concentration was calculated as LC50X0.1.
A one-way ANOVA, followed by Duncan’s multiple comparison procedure, was used to determine significant differences in LOI in sediment and in TOC, TN, and NH4+ concentrations in seawater at different elapsed times.
>
Acute peptone toxicity tests
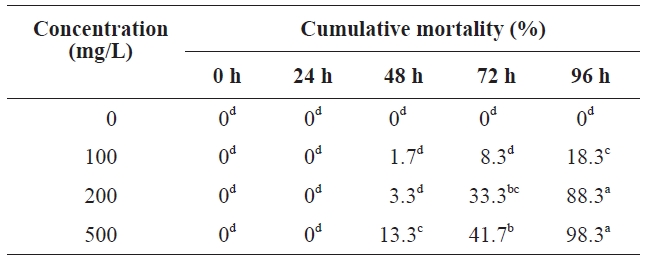
Table 1 summarizes our results. No polychaetes died at the earliest time point. At 48 h, mortalities in different concentrations were still low, with mortality at 300 mg/L being 13.3%, which differed significantly from mortalities at other concentrations (ANOVA,
[Table 1.] Toxicity of peptone to polychaeta
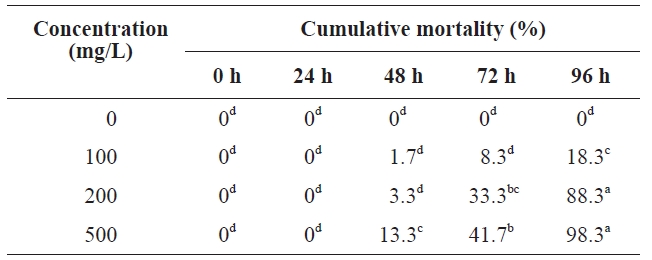
Toxicity of peptone to polychaeta
increased sharply, with no significant differences (
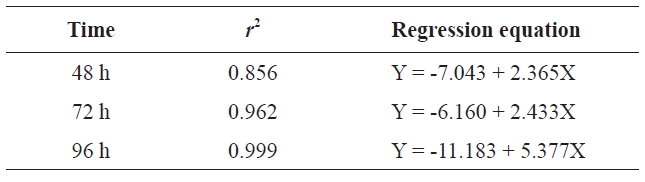
Probit analysis comparing concentration and cumulative mortality was conducted using SPSS software version 18.0 (IBM, Yorktown Heights, NY, USA). Mortality probability was linearly dependent on log concentration (Table 2). The
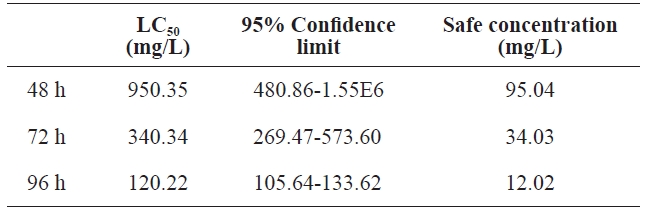
Using regression equations, we calculated LC50 and the 95%confidence limit at different times. Table 3 shows the results. At 48 h, 72 h, and 96 h, LC50 was 950.35, 340.34, and 120.22 mg/L, respectively. At 96 h, the 95% confidence limit was statistically significant at 105.64-133.62. The safe concentration was 12.02 mg/L.
>
Sediment analysis of long-term exposure experiment
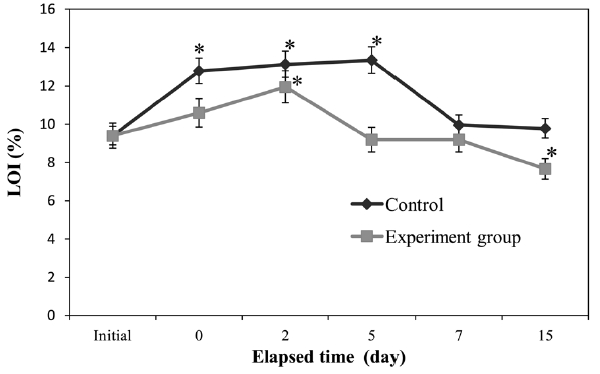
Fig. 1 shows the trends in LOI. Control sediment LOI was elevated significantly (
>
Seawater analysis of the long-term exposure experiment
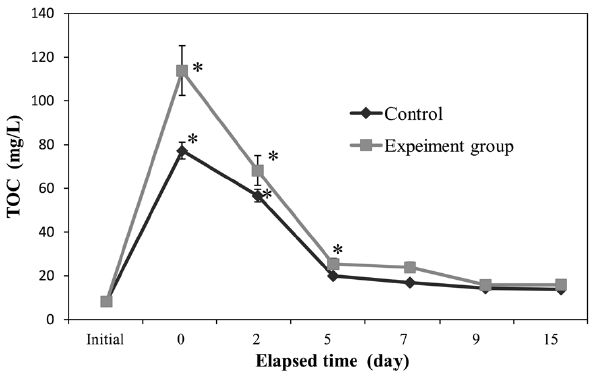
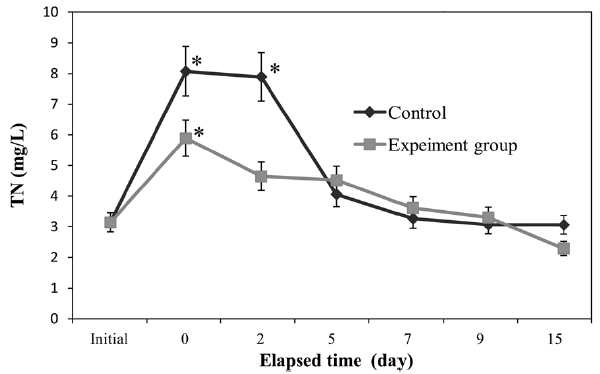
We monitored seawater TOC and TN in both control and experimental groups (Figs. 2 and 3). In both groups, TOC and TN increased significantly (
[Table 2.] Regression equation at different times
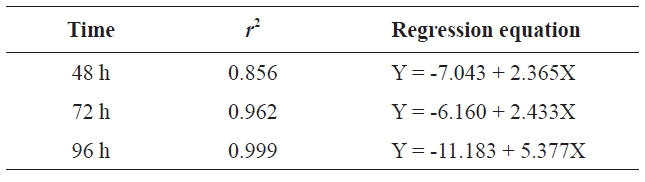
Regression equation at different times
[Table 3.] The LC50 and safe concentration of peptone for the polychaeta
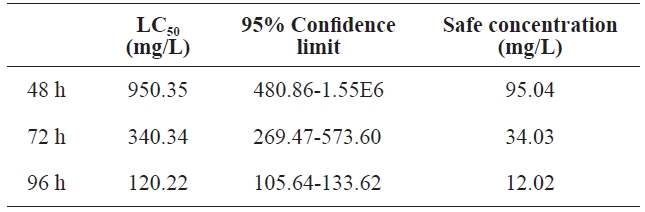
The LC50 and safe concentration of peptone for the polychaeta
of TOC and TN in the experimental group was greater than that of the control group.
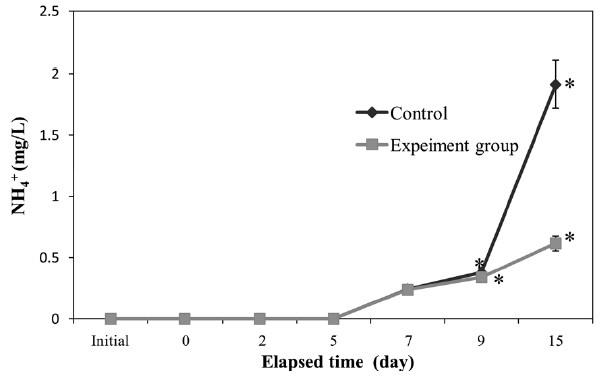
The concentration in seawater of NH4+ (Fig. 4) increased with time. At 9 days, a significant difference (
apparent in both groups. However, the final NH4+ concentration in the control was significantly higher (
>
Sediment and water improvement
High levels of peptone, which is a source of organics and nitrogen, can lead to eutrophication (Shikano and Kawabata, 2000). Thus, the addition of synthetic eutrophic seawater to the experimental group resulted in a significant increase in sediment LOI, as well as seawater TOC and TN (Figs. 1-3). However, in the experimental group, LOI and TN increments were lower than those of the control and had decreased below initial levels by the end of the experiment. Our results indicate that polychaetes can accelerate the recovery of sediments and seawater from eutrophication. Polychaetes have been shown to improve sediment conditions in scallop aquaculture (Kang et al., 2010). As a deposit-feeder, polychaetes ingest sediment and digest organic matter in the sediment, before defecating the altered sediment. Some research has indicated that the digestive fluids of deposit-feeders are more effective than water at solubilizing organic materials associated with sediments (Ahrens et al., 2001). In addition, disturbances caused by polychaetes aid the growth of specific bacterial groups, which break down, use, and enrich specific types of organic matter (Cuny et al., 2007). These two mechanisms may explain how polychaetes can reduce organics in seawater and sediment.
Peptone-rich peptide can be reduced to toxic ammonia and nitride by the activities of microorganisms (Shikano and Kurihara, 1988). Few studies have investigated the toxicity of peptone, in contrast to the plethora of studies on ammonia toxicity. Ammonia is acutely toxic to marine animals. Chronic exposure of fishes to sublethal levels of ammonia causes reduced food intake and slower growth (Pinto et al., 2007). Endocrine system functions appear to be especially affected in marine animals (Spencer et al., 2008). Elevated concentrations of ammonia in the surrounding water may reduce or prevent ammonia excretion by fishes, leading to a buildup of ammonia in the plasma and ultimately leading to death (Brinkman et al., 2009). In cobia, the 96-h LC50 of ammonia has been estimated at 1.13 mg/L (Rodrigues et al., 2007), and that of cutthroat trout is about 0.3-0.8 mg/L (Thurston et al., 1978). During our acute toxicity bioassays, the LC50 of peptone was 120.22 mg/L, and even when 1%of the peptone was reduced, the LC50 ammonia concentration was greater than that of the two species above. The ability of polychaetes to exist in highly eutrophic areas lends support to the idea that polychaeta can improve eutrophic environments.
The NH4+ concentration in seawater increased with time (Fig. 4), which accords with a previous study showing that peptone-rich peptides can be reduced to ammonia (Shikano and Kurihara, 1988). We found that polychaetes slowed the increase in NH4+ compared to controls. However, the NH4+ concentration still increased with time, reaching 0.5 mg/L, which could impact other species. To control the increase in NH4+, integrated bioremediation should be applied, perhaps using nitrifying bacteria (Yang et al., 1999).
In summary, we found that, under lab conditions, polychaetes can be used to alleviate eutrophication. Further research should be conducted to determine whether our data are applicable to real environmental conditions.