The antioxidant activities of a methanolic extract of Eisenia bicyclis and its organic solvent fractions, including dichloromethane (CH2Cl2), ethyl acetate (EtOAc), n-butanol (n-BuOH), and water (H2O) fractions, were investigated. Scavenging activities against DPPH, hydroxyl, superoxide anion, and peroxynitrite radicals were evaluated using electron spin resonance spectrometry; intracellular reactive oxygen species (ROS) were evaluated by a 2′,7′-dichlorofluorescein diacetate assay using RAW264.7 mouse macrophages. The antioxidant activities of the individual fractions were: EtOAc>n-BuOH>CH2Cl2>H2O. The EtOAc fraction exhibited strong radical scavenging activity and a significantly reduced ROS level in RAW264.7 cells. Moreover, the phenolic contents of the extract and fractions followed the same order as their radical scavenging activities. Our results indicate that E. bicyclis is a valuable natural source of antioxidants that may be applicable to the functional food industry.
Free radicals are produced by endogenous factors, such as normal respiration, and exogenous factors, such as the metabolism of foreign materials, smoking, and ultraviolet (UV) radiation (Pryor, 1986; Robinson et al., 1997). Inspired molecular oxygen reacts readily with free radicals to generate reactive oxygen species (ROS), including superoxide anion radical, hydroxyl radical, singlet oxygen, and hydrogen peroxide (H2O2), as well as reactive nitrogen species (RNS), including peroxynitrite, formed by the reaction of nitric oxide and superoxide anion in the body (Sawa et al., 2000; Yildirim et al., 2000). These ROS and RNS can cause oxidative damage to several cellular components, including lipids, proteins, and nucleic acids, and induce inflammation or lesions in various organs (Beckman et al., 1990). These reactive species are also associated with various degenerative diseases, including cancer, aging, arteriosclerosis, rheumatoid arthritis, and allergy (Dreher and Junod, 1996; Griffiths and Lunec, 1996; Squadrito and Pryor, 1998; Sohal, 2002).
Recently, there has been great interest in marine resources as a source of powerful and nontoxic natural antioxidants, given their reported inhibition of oxidative stress and aging-induced disorders. Numerous crude extracts and pure compounds obtained from marine resources have been reported to possess antioxidant and radical scavenging activities (Lim et al., 2002; Kang et al., 2004; Kuda et al., 2005; Duan et al., 2006; Ganesan et al., 2008; Zou et al., 2008). In particular, marine algae are of great interest due to their diverse natural products, unique structure, and biological abilities based on their antioxidant activity.
>
Preparation of the MeOH extract and fractions
The lyophilized powder of
>
DPPH radical scavenging activity assay
DPPH radical scavenging activity was measured using the method of Nanjo et al. (1996). A 30 μL sample (or ethanol itself as a control) was added to 30 μL of DPPH (60 μM) in ethanol. After mixing vigorously for 10 s, the solution was transferred to a 100 μL quartz capillary tube and the scavenging activity of the samples toward DPPH radicals was measured using a JESFA electron spin resonance (ESR) spectrometer (JEOL, Tokyo, Japan). The spin adduct was measured on an ESR spectrometer exactly 2 min later. The experimental conditions were: magnetic field, 336.5±5 mT; power, 5 mW; modulation frequency, 9.41 GHz; amplitude, 1Χ1,000; and sweep time, 30 s. The DPPH radical scavenging ability of each sample was calculated according to the following equation:
DPPH scavenging activity (%)=(1-A/A0)Χ100,
where
>
Hydroxyl radical scavenging activity assay
Hydroxyl radicals were generated by the iron-catalyzed Fenton Haber-Weiss reaction, and the radicals were rapidly reacted with a nitrone spin trap using DMPO. The resultant DMPO-OH adducts were detected with an ESR spectrometer (Rosen and Rauckman, 1984). The sample solution (20 μL) was mixed with DMPO (0.3 M, 20 μL), FeSO4 (10 mM, 20 μL), and H2O2 (10 mM, 20 μL) in a phosphate buffer solution (pH 7.4) and then transferred to a 100 μL quartz capillary tube. After 2.5 min, the ESR spectrum was recorded using an ESR spectrometer. The experimental conditions were: magnetic field, 336.5±5 mT; power, 1 mW; modulation frequency, 9.41 GHz; amplitude, 1Χ200; and sweep time, 4 min. The scavenging activity was calculated as follows:
Hydroxyl radical scavenging activity (%)=(1-A/A0)Χ100,
where
>
Superoxide radical scavenging activity assay
Superoxide radicals were generated using a UV-irradiated riboflavin/EDTA system (Guo et al., 1999). The reaction mixture containing 0.3 mM riboflavin, 1.6 mM EDTA, 800 mM DMPO and the indicated sample concentration was irradiated for 1 min under a UV lamp at 365 nm. The reaction mixture was then transferred to a 100 μL quartz capillary tube for measurement by ESR spectrometry. The experimental conditions were: magnetic field, 336.5±5 mT; power, 10 mW; modulation frequency,9.41 GHz; amplitude, 1Χ1,000; and sweep time, 1 min. The superoxide radical scavenging ability of each sample was calculated as follows:
Superoxide radical scavenging activity (%)=(1-A/A0)Χ100,
where
>
Peroxynitrite scavenging activity assay
Peroxynitrite scavenging activity was assessed according to a modified version of Kooy’s method (Kooy et al., 1994). Highly fluorescent rhodamine 123, which is rapidly oxidized from non-fluorescent DHR 123 in the presence of peroxynitrite, was monitored. In brief, the rhodamine buffer (pH 7.4) used in this assay consisted of 50 mM sodium phosphate dibasic, 50 mM sodium phosphate monobasic, 90 mM sodium chloride, 5 mM potassium chloride, and 100 μM diethylenetriaminepentaacetic acid. The final DHR 123 concentration was 5 μM. The buffer used in this assay was freshly prepared and maintained on ice. The samples were dissolved in 10% dimethyl sulfoxide (DMSO) at 12.5-100 μg/mL for the extract/fractions. The fluorescence intensity of the oxidized DHR 123 was evaluated using a GENios microplate reader (Tecan Austria GmbH, GrOdig, Austria) at excitation and emission wavelengths of 480 and 530 nm, respectively. All values are expressed as the mean±SD of three experiments.
>
Quantification of the total phenolic content
The total phenolic contents were determined via a modified version of the Folin-Ciocalteu method using gallic acid as a standard (Singleton and Rossi, 1965). A 0.1 mL aliquot of the extract was mixed with 1 mL of Folin-Ciocalteu reagent (previously diluted 1:1 [v/v] with water and 2 mL of 20% sodium carbonate [Na2CO3] solution). The mixed solution was maintained at room temperature for 45 min, followed by 10 min of centrifugation at 5,000 rpm. The absorbance of the supernatant was measured at 730 nm using a GENios microplate reader (Tecan Austria GmbH). The total phenolic contents of the fractions are expressed as a percentage (wt %) compared to the weight of the dried extract or fraction.
RAW264.7 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heated-inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37℃ in a humidified atmosphere of 95% air and 5% CO2. The medium was changed every other day.
>
Assessment of cell cytotoxicity
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases (Mosmann, 1983). Cells were cultured in 96 well plates (1.5 Χ 105 cells/well) with serum free media and treated with different concentrations of sample for 24 h. The MeOH extract of
>
Measurement of the intracellular ROS-scavenging activity using DCFH-DA labeling
To assess intracellular ROS formation, the redox sensitive fluorescent probe DCFH-DA was used as described by LeBel et al. (1992). RAW264.7 cells were incubated with 50 μM DCFH-DA for 30 min at 37℃ in the dark. Following three washes with PBS, the cells were incubated with different samples (100 μg/mL) for 1 h then mixed with 500 μM H2O2. The level of intracellular ROS in the collected cells was determined using a FACSCalibur™ flow cytometer (488 nm excitation, 530 nm emission) equipped with CELLQUEST analysis software (Becton Dickinson, Mountain View, CA, USA). For each treatment, 10,000 cells were counted. The results are expressed as the average H2DCF-DA fluorescence intensity in the cells to determine the free radical scavenging activities of the samples.
In the present study, we focused on the antioxidant effects of a MeOH extract of
Free radicals and their major ROS are unstable and react with various macromolecules in the human body (Halliwell and Gutteridge, 1990). In fact, free radicals and ROS or RNS, including hydroxyl radical, superoxide anion and peroxynitrite, play a crucial role in the etiology of several diseases (Beckman et al., 1990), including cancer, Alzheimer’s disease, rheumatoid arthritis, and atherosclerosis (Squadrito and Pryor, 1998). Thus, the scavenging of free radicals and ROS is probably one of the most effective defenses in the body against disease. ESR spectroscopy is used to evaluate chemical species such as free radicals or inorganic complexes possessing a transition metal ion. In recent years, ESR has been widely used to measure free radical levels in radical scavenging activity assays due to the convenience and high sensitivity of the technique (Sachindra et al., 2007).
>
DPPH radical scavenging activity
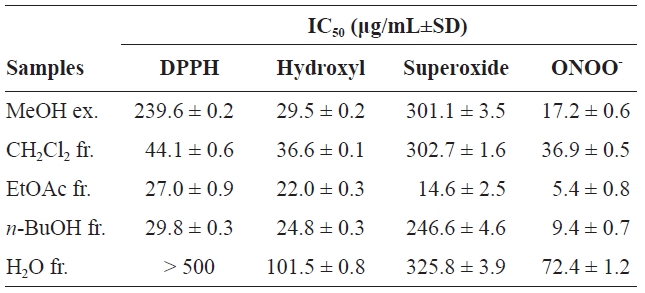
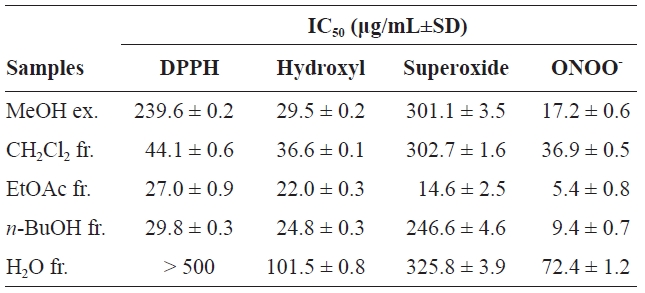
DPPH is a stable free radical that has been extensively used to assess the antioxidative activities of antioxidants (Antolovich et al., 2002). As summarized in Table 1, the scavenging activity of the MeOH extract (ex.) and its solvent soluble fractions (fr.) toward DPPH radicals was as follows: EtOAc fr.>
>
Hydroxyl radical scavenging activity
Hydroxyl radicals are the shortest lived and most highly reactive ROS; they react rapidly with biological molecules and can attack cellular molecules, including hepatic tissue (Hippeli and Elstner, 1997). Hydroxyl radicals were generated by the Fenton reaction (Fe2++H2O2→ Fe3++OH-+·OH) and trapped by a stable radical, DMPO, forming nitroxide adducts that were detected by ESR spectrometry (Makino et al., 1991). As shown in Table 1, the extract and fractions exhibited potent or moderate scavenging activity against hydroxyl radicals. The order of the IC50 values for the extract and fractions was: EtOAc fr. (22.0±0.3 μg/mL)>
>
Superoxide anion scavenging activity
Radical scavenging effects of the MeOH extract (ex.) of Eisenia bicyclis and its solvent fractions (fr.)
Superoxide anion is a precursor of single oxygen and hydroxyl radicals and a weak oxidant that indirectly initiates lipid peroxidation. The presence of superoxide anion can magnify cellular damage because it increases other free radicals and oxidizing agents (Zou et al., 2008). The superoxide anion scavenging activity of the extract and fractions are shown in Table 1. The potential scavenging activity of the extract and fractions were: EtOAc fr.>
>
Peroxynitrite scavenging activity
Peroxynitrite, formed from the reaction of superoxide with nitric oxide, is cytotoxic (Squadrito and Pryor, 1998). The need for a strong peroxynitrite scavenger is clear due to the absence of an enzyme that can exert protective effects against damage induced by peroxynitrite. As shown in Table 1, the IC50 values were found to be 17.2±0.6, 36.9±0.5, 5.4±0.8, 9.4±0.7, and 72.4±1.2 μg/mL for the MeOH ex., CH2Cl2 fr., EtOAc fr.,
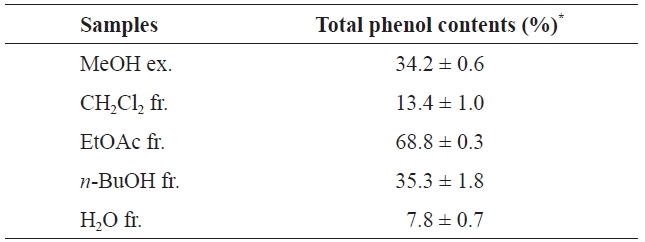
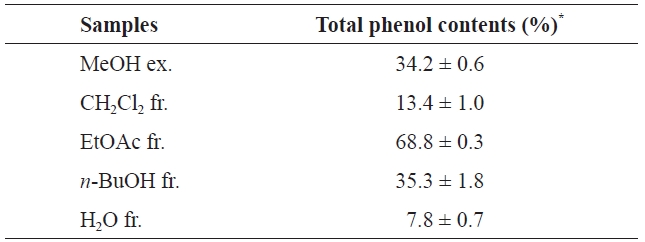
Phenolic compounds are common in plants and have been reported to have various bioactivities, including antioxidant activities. According to previous studies, marine algae and their polyphenols have antioxidant properties (Yan et al., 1999; Lim et al., 2002; Kuda et al., 2005). The major active polyphenols in algae extract are phlorotannins (Yan et al., 1999). The total phenolic contents of the MeOH extract of
Total phenolic contents of the MeOH extract (ex.) of Eisenia bicyclis and its solvent fractions (fr.)
(34.2±0.6%), CH2Cl2 fr. (13.4±1.0%), and H2O fr. (7.8±0.7%). Notably, the order of the total phenolic contents is similar to that of antioxidant activities. Several studies have shown a close relationship between antioxidant activity and the total phenolic content (Negro et al., 2003; Toor and Savage, 2005).
>
Cytotoxicity of the extract and fractions toward RAW264.7 cells
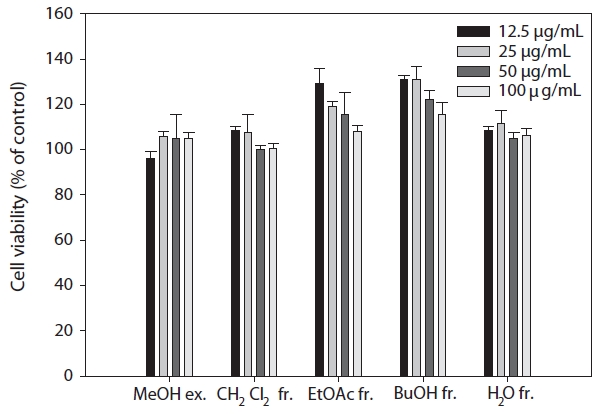
The cytotoxicities of the tested extract and fractions were assessed using RAW264.7 cells to evaluate the endocellular action of antioxidation. Our results show that the tested extract and fractions had no cytotoxic effects, even at a concentration of 100 μg/mL. In addition, a marked difference could not be found between the tested extract and fractions and the control (Fig. 1).
>
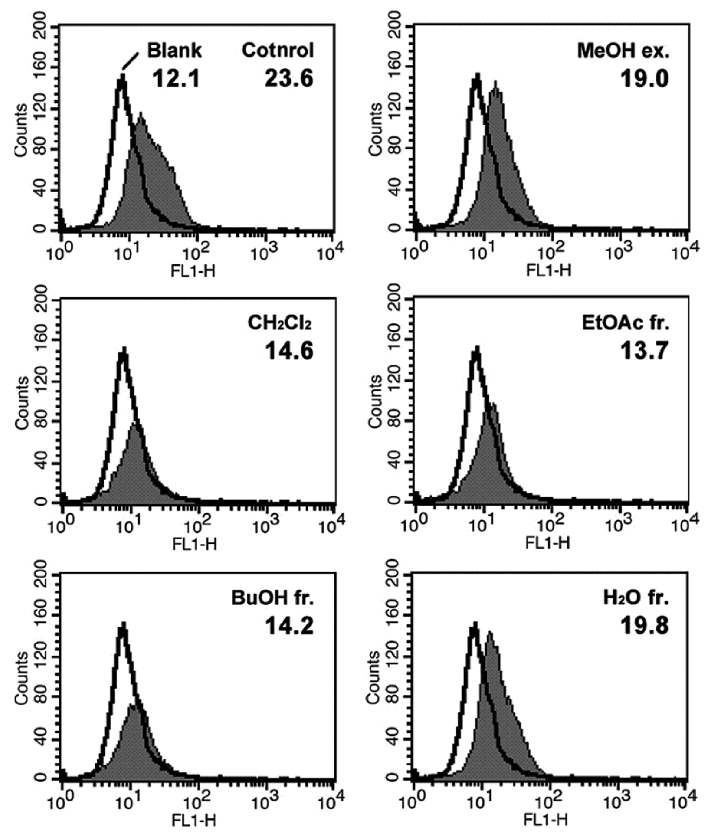
Cellular ROS determination using DCFH-DA
To evaluate the direct radical scavenging effects of the MeOH extract and its solvent fractions in cells, DCFH-DA was used as the substrate to measure intracellular ROS production in neutrophils. DCFH-DA freely penetrates into cells and is hydrolyzed to dichlorofluorescin (DCFH) by intracellular esterases. This non-fluorescent dye is then oxidized to fluorescent dichlorofluorescin (DCF) by the action of cellular ROS. RAW264.7 cells were selected to investigate the effects of phlorotannins in the MeOH extract on the intracellular production of ROS. As shown in Fig. 2, the average DCF fluorescence in the control, which was treated with 500 μM H2O2, was twice (23.6) that in the blank (H2O2 non-treated) after 2 h. However, pretreatment with the MeOH extract and its solvent fractions decreased the average DCF fluorescence, as compared to the control. This result indicates that the MeOH extract and its solvent fractions had considerable intracellular radical scavenging activities in RAW264.7 cells. As with our other radical scavenging assay, the MeOH extract and H2O fraction showed moderate radical scavenging activities while the EtOAc fraction exhibited the highest radical scavenging activity (13.7). This result suggests that the radical scavenging activity of the EtOAc fraction, which had the highest total phenolic content (68.8±0.3%) (Table 2), was caused by phlorotannins. Together, our results suggest that the EtOAc fraction is a potent antioxidant material that can protect against the radical-mediated oxidation of cellular biomolecules and that contains various antioxidative phenolic compounds, including phlorotannins.
The present study demonstrated that a MeOH extract of