Cytoskeletal changes were observed during cell division of the green alga Zygnema cruciatum using flourescein isothiocynate(FITC)-conjugated phallacidin for F-actin staining and FITC-anti-α-tubulin for microtubule staining. Z. cruciatum was uninucleate with two star-shaped chloroplasts. Nuclear division and cell plate formation occurred prior to chloroplast division. Actin filaments appeared on the chromosome and nuclear surface during prophase, and the F-actin ring appeared as the cleavage furrow developed. FITC-phallacidin revealed that actin filaments were attached to the chromosomes during metaphase. The F-actin ring disappeared at late metaphase. At telophase, FITC-phallacidin staining of actin filaments disappeared. FITC-anti-α-tubulin staining revealed that microtubules were arranged beneath the protoplasm during interphase and then localized on the nuclear region at prophase, and that the mitotic spindle was formed during metaphase. The microtubules appeared between dividing chloroplasts. The results indicate that a coordination of actin filaments and microtubules might be necessary for nuclear division and chromosome movement in Z. cruciatum.
Since the first observation of microfilaments in a freshwater alga,
Microfilaments are thought to play a key role in cytokinesis furrowing, while not being involved in nuclear division. Sampson and Pickett-Heaps (2001) observed microfilaments associated with chromosomes, often in paired spots, in
In this study, we observed the cytoskeletal changes during cell division of the green alga
>
Plant material and laboratory culture
Algal materials were collected from ponds in Kongju, Korea, from December, 2004 to January, 2005. The plants were washed three times with Bolds basal medium (Bischoff and Bold 1963) and kept in the same medium at 4°C, using a 12 : 12 hour light : dark cycle with cool-white fluorescent lighting (> 20 ㎛ol photons m-2s-1). Germanium dioxide was added to the medium (final concentration 1 mg L-1) for 2 weeks to eliminate diatoms.
>
Fluorescence localization of actin and DNA
To visualize the actin cytoskeleton,
>
Fluorescence localization of microtubules
For time-lapse video-microscopy, the filaments were placed on a glass slide and a coverslip was applied and sealed with a 1 : 1 : 1 mixture of Vaseline, lanolin and paraffin that had been prepared by melting on a hot plate at 70oC. The slide preparations were examined using an Olympus BX-51 microscope under the oil immersion ×20 objective lens, and the images were recorded using a Digital Imaging Time-Lapse Recorder (TCS Korea, Daejeon, Korea).
>
Observation of cell division using time-lapse video-microscopy
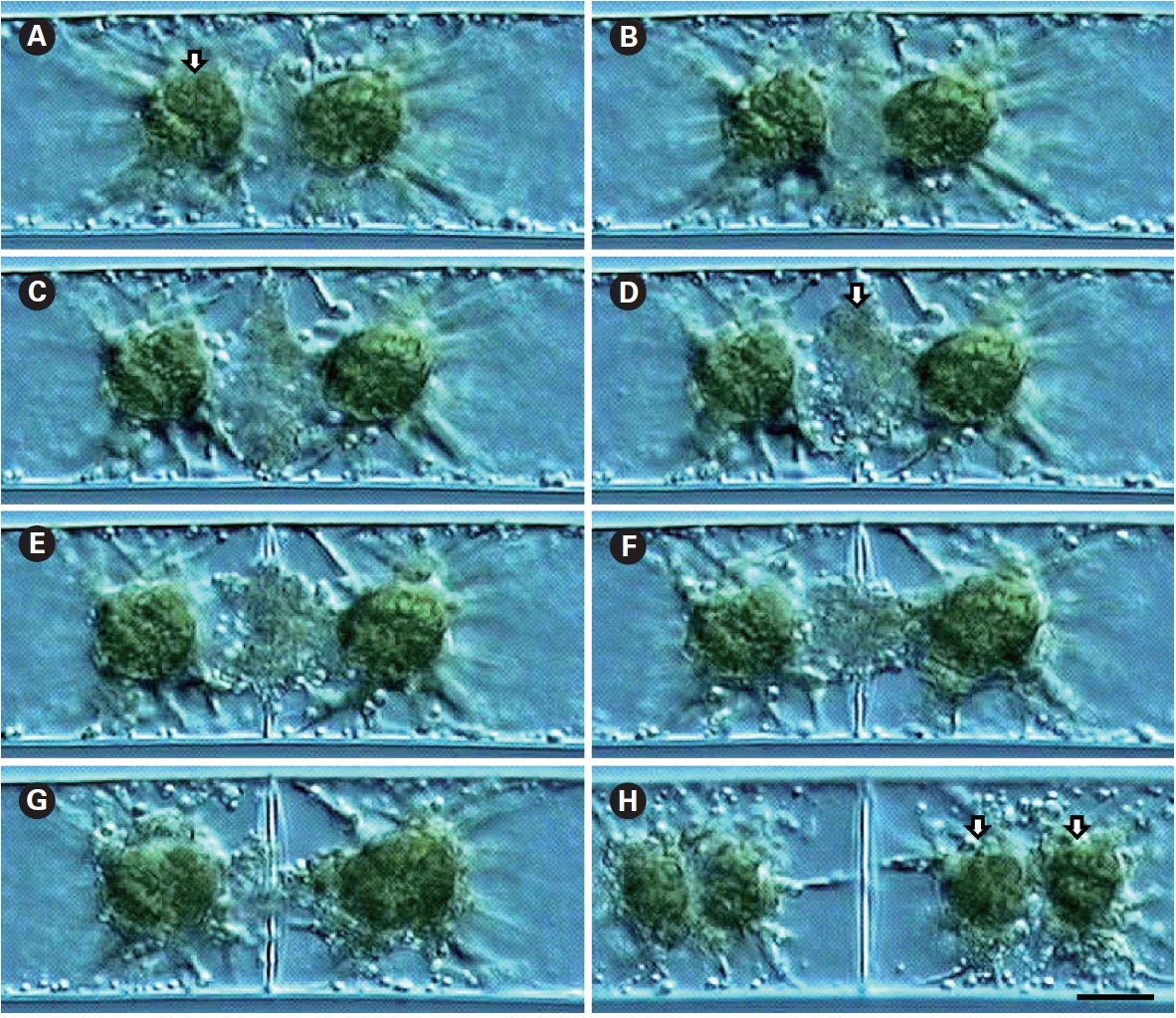
Cell division was observed using time-lapse video-microscopy. Cells were uninucleate and possessed two star-shaped chloroplasts (Fig. 1A). Cell division always proceeded simultaneously with nuclear division, with the cell wall first discerned at the cell periphery, with development towards the cell center (Fig. 1B). Vesicles involved in cell plate formation decreased with the development of the cell wall (Fig. 1C-G). Usually, chloroplast division followed nuclear division and cell plate formation (Fig. 1H).
Fluorescence localization of actin and DNA
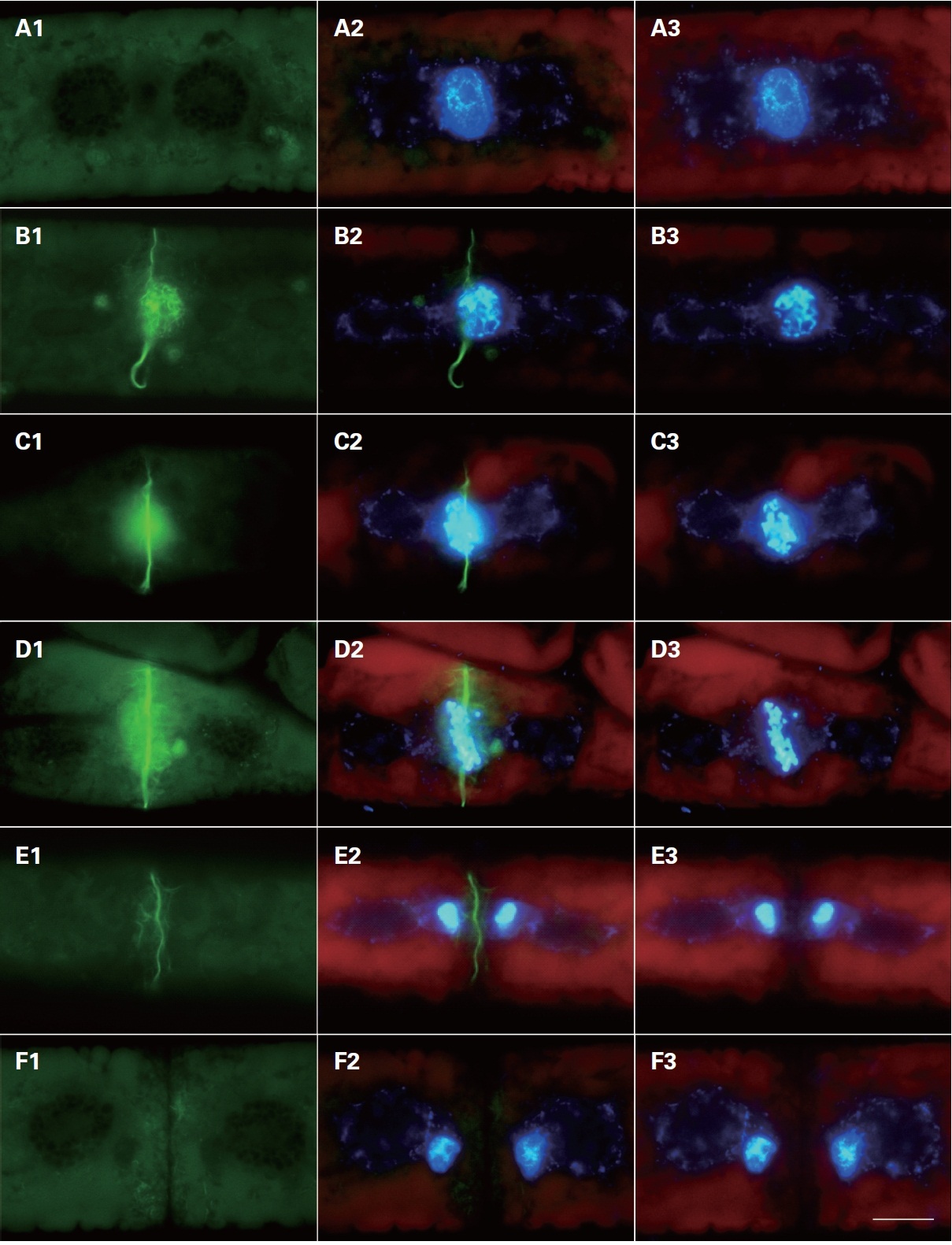
Cytoskeletal changes were observed during cell division using FITC-phallacidin for F-actin staining. Microfilaments began to appear at the center of the division plate when nuclear division started (Fig. 2). These filaments were not visible in control cells pretreated with non-fluorescent phallacidin (data not shown). Therefore, filaments revealed using FITC-phallacidin presumably represented cytoskeletal microfilament bundles composed of F-actin.
In interphase cells, F-actin was randomly distributed in the cytoplasm near the cell surface. Normally, the actin filaments were not observed in the centers of the cells (Fig. 2A1). In prophase, the nuclear envelope was broken down; two typical forms of staining were observed in different locations of the cell. The first type of staining was a fibrous web around the nucleus, in which F-actin tightly surrounded the prophase nucleus forming a finely interwoven net-like cage as apparent in the surface view (Fig. 2B1). In the other type of staining, the F-actin was evident at the middle of the cells forming a fibril ring situated just beneath the plasma membrane (Fig. 2C1 & D1). A faint trace of protoplast-furrowing was visible in mid-
prophase cells. These protoplast-furrowing structures were tightly associated with the fibril ring (Fig. 2C1).
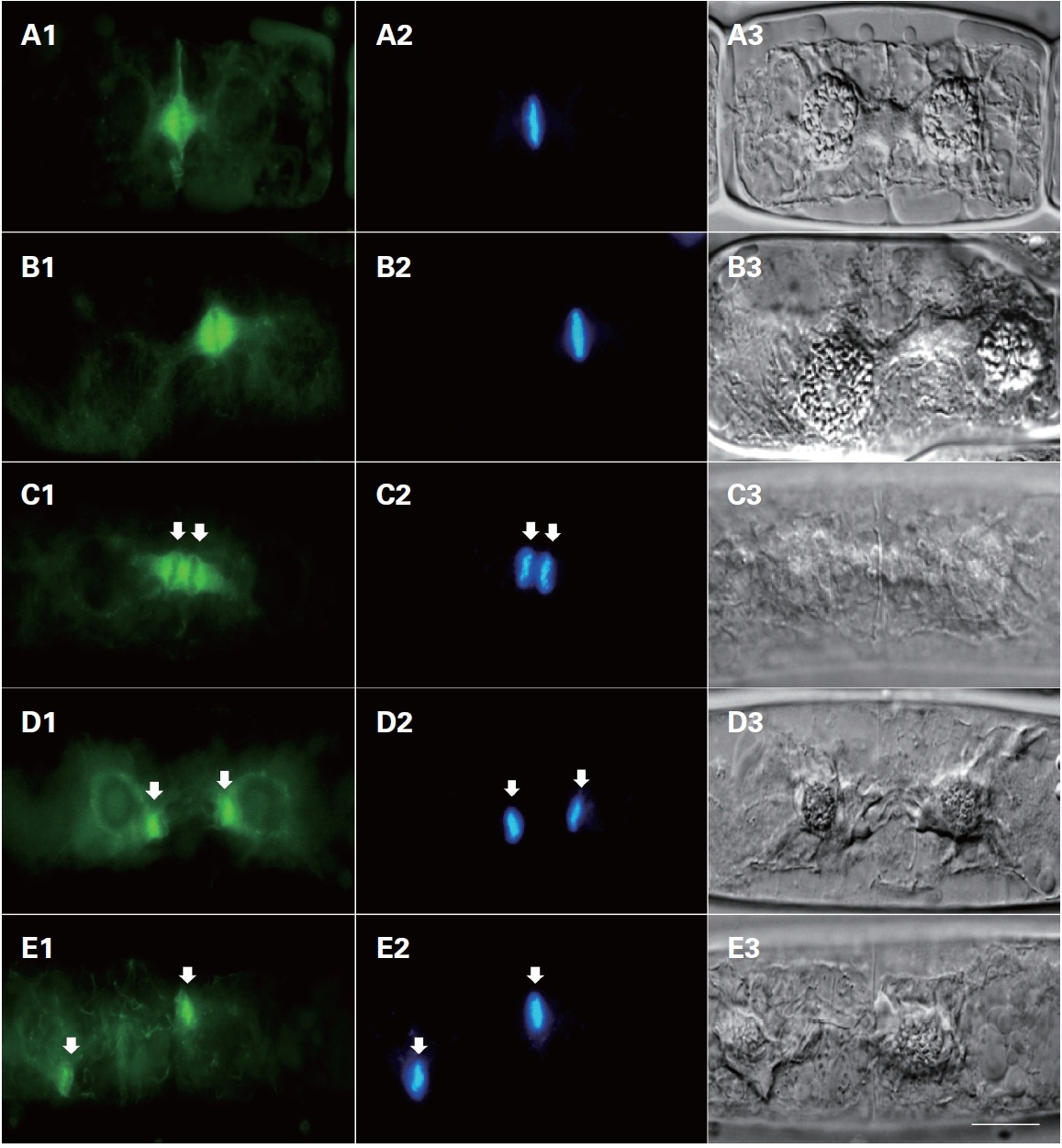
In metaphase cells, the chromosomes were aligned at the cell equator, and the net-like cage of F-actins ran nearly parallel with the individual chromosomes, but ultimately most formed a spindle located on the individual chromosomes (Fig. 2D1, 3A1 & B1). In this stage, the fibril rings were similar in appearance to those in prophase cells (Fig. 2B1 & C1). The diameter of the fibril ring
continuously decreased with the development of the furrow, until each cell divided into two daughter cells (Fig. 2D1-F1).
In anaphase cells, the duplicated chromosomes separated, and the spindle form of F-actins associated with the daughter chromosomes moved from the equator of the cell to spindle poles (Fig. 3C1 & D1). The spindle form of F-actins was similar in appearance to those in metaphase cells (Fig. 3E1). The F-actins were localized at the spindle pole side in late anaphase. Furrowing of the protoplast was most conspicuous at this stage. A fibril ring was not detected in the same area at anaphase.
In telophase cells, the daughter chromosomes reached to the mitotic poles, and actin filaments were observed on the daughter nuclei (Fig. 3E1 & E2). These F-actins tightly surrounded the telophase nucleus and continuously decreased with the completion of cell division. Different types of F-actins were evident in the cytoplasm near the surface of the cell, most of them running irregularly (Fig. 3E1).
>
Fluorescence localization of microtubules and DNA
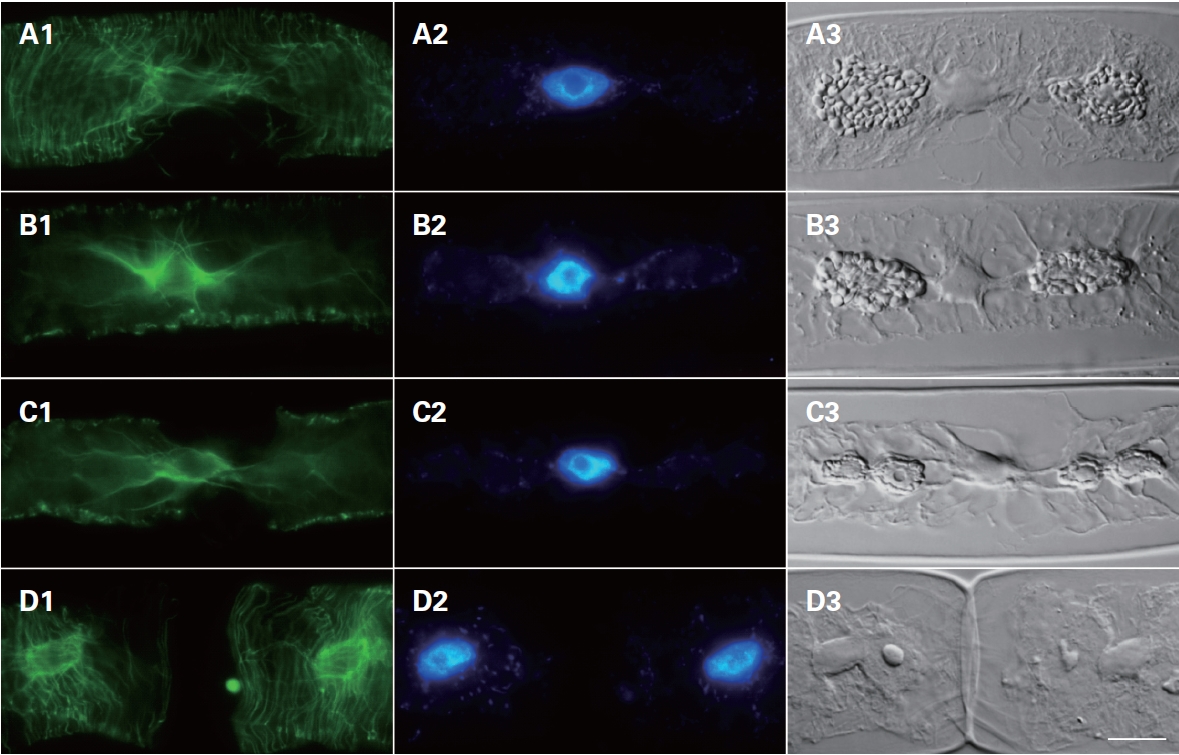
Cytoskeletal changes were observed during cell division using FITC-anti-α-tubulin to stain microtubules. In interphase cells, the microtubules were arranged beneath the protoplasm of the cells, most of them running nearly parallel to the cross-wall surrounding the nucleus (Fig. 4). In cells progressing from prophase to meta-
phase, microtubules were localized at the nuclear region at prophase (Fig. 4B1) but became an organized mitotic spindle at metaphase (Fig. 4B1). Interestingly, microtubules appeared between dividing chloroplasts (Fig. 4C1 & C3). Finally, after forming the new cell wall (Fig. 4D3), microtubules were re-arranged beneath the cell wall (Fig. 4D1).
The present study recorded the following observations. During interphase, actin filaments were located along the cell surface running parallel to the long axis of the cell. During prophase, a fibrous web of F-actin appeared around the nucleus and a fibril ring was formed at the division center just beneath the plasma membrane. The F-actins of metaphase cells co-aligned with spindle microtubules to contact individual chromosomes. The spindle form of the F-actin was associated with daughter chromosomes moving from the equator of the cell to spindle poles during anaphase. Finally, during telophase, F-actins tightly surrounded the nucleus. The amount of F-actin in the center of cell continuously decreased with the completion of cell division. These data provided the first comprehensive evidence suggesting a coordinate involvement of microfilaments and microtubules during nuclear division in
The F-actins apparent during interphase appeared in the cytoplasm near the surface of the cell, and disappeared with the progress of cytokinesis. They might be involved in vesicle and organelle transport, either alone or with the microtubules. They could also be responsible for cytoplasmic streaming, although this function was not evident in
Four types of microfilaments have been reported in
The organization of F-actin in the spindle of