Diatoms of the family Rhizosoleniaceae De Toni are an important group of marine phytoplankton, which are obtained from net and water samples from a variety of habitats. The genera
In coastal waters of Korea, Shim (1994) reported 26
The present study was undertaken to provide a detailed survey of the genus
To remove cell organelles and organic matter, samples were prepared as previously described (Hasle and Fryxell 1970, Simonsen 1974) with some modifications. Briefly, each sample was extensively washed with distilled water using five cycles of centrifugation followed by decantation of as much water as possible, leaving the sample almost dry. An equal amount of saturated KMnO4 was added, and the sample was dispersed by agitation and left for 24 h. An equal volume of concentrated HCl was added, the sample was boiled gently, and rinsed / decanted repeatidly with distilled water as before.
LM observations of acid-cleaned natural samples were conducted using an Axioskop 40 light microscope (Carl Zeiss, Jena, Germany) equipped with a Axiocam MRc5 digital camera (Carl Zeiss). The cleaned materials were mounted to produce a permanent slide as previously described (Hasle and Fryxell 1970). Briefly, a drop of each cleaned sample was transferred to a cover slip that was then cleaned with 100% ethanol. The deposited material was dried over a gentle heat or left overnight until the resin became firm. Dehydrated sub-samples were immersed in Pleurax. The permanent slides were observed at a magnification of ×400 or ×1,000.
>
Scanning electron microscopy
SEM was done using a model JSM-5600LV apparatus (Jeol, Tokyo, Japan). Acid-cleaned natural samples, or samples prepared as previously described (Hasle and Fryxell 1970) were attached to aluminum stubs and coated with cold-palladium. Other samples were prepared as described by Jung et al. (2010), where live cells were fixed with modified Parducz’s fixative (Parducz 1967) at 1 : 1 v/v for 10 min at room temperature. The fixative was a 2% solution of osmium tetroxide in filtered seawater and a saturated solution of mercuric chloride in distilled water mixed at a ratio of 5 : 1 v/v, respectively. Fixed cells were harvested by gravity filtration on a 2.0 ㎛ polycarbonate membrane (TTTP; Millipore, Billerica, MA, USA). To prevent the formation of NaCl crystals, any seawater remaining associated with the specimen was removed by washing for 2 min at room temperature with drops of distilled water followed by drops of 0.05 M C2H12AsNaO5 buffer (pH 8.0). For dehydration, drops of diethyl ether (C4H10O) were continuously dripped on the samples for 10 min at 30°C. Following this procedure, several drops of hexamethyldisilane (C6H19NSi2) were immediately dispensed on the membrane to complete the drying process.
>
Taxonomic identification and terminology
Monographs of species belonging to family Rhizosoleniaceae were used for identification (Cupp 1943, Hendey 1964, Sundstrom 1986, Hasle and Syvertsen 1996). Terminology followed previously published general proposals (Ross et al. 1979, Sundstrom 1986, Round et al. 1990, Hernandez-Becerril 1995, Hasle and Syvertsen 1996).
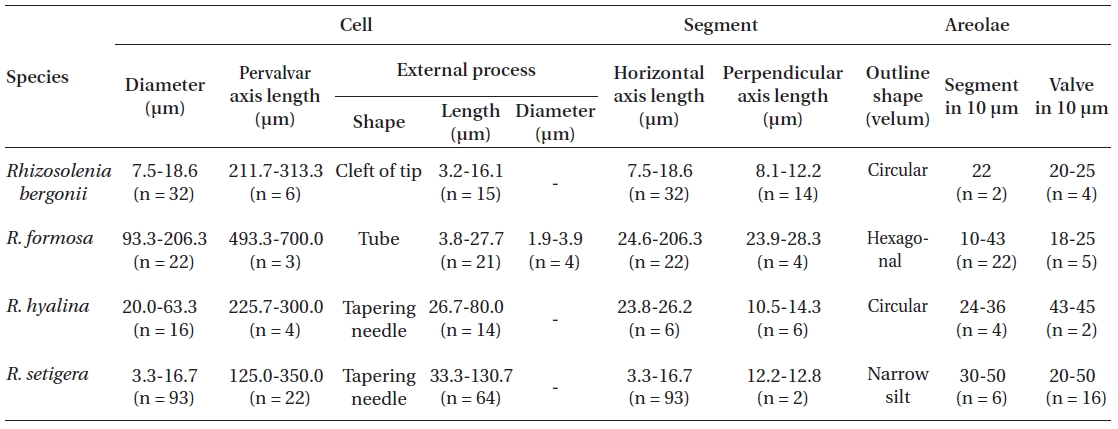
Systematics and phylogenetics of members of the family Rhizosoleniaceae are suggested by Sundstrom (1986) as follows. The characters observed in the Rhizosolenia species presently-studied are summarized in Table 2. Each description includes references for identification,
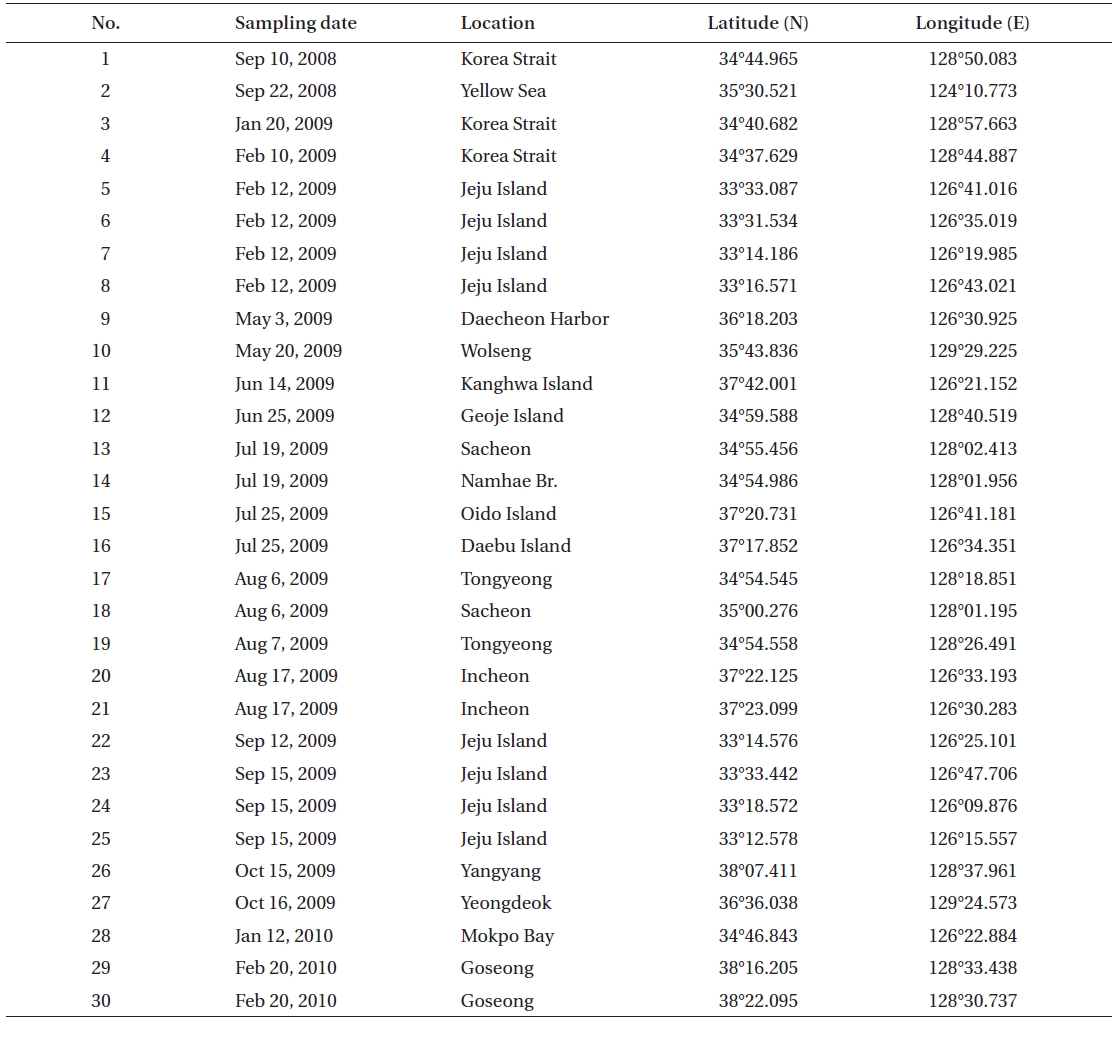
[Table 1.] Collection site information of the Rhizosolenia specimens examined in the present study
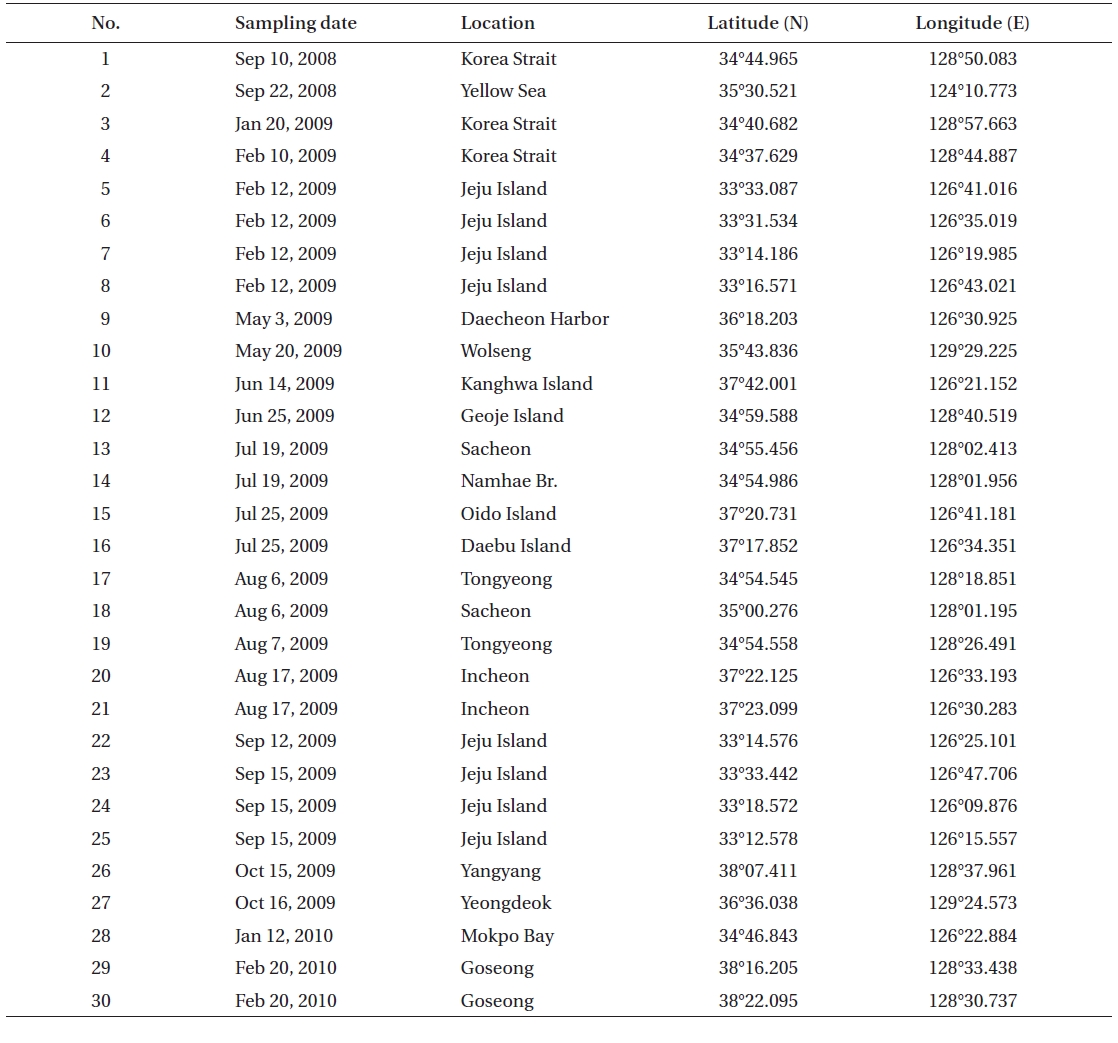
Collection site information of the Rhizosolenia specimens examined in the present study
by means of LM and SEM.
Class Bacillariophyceae Haeckel 1878
Order Centrales Hustedt 1930
Suborder Rhizosoleniineae Simonsen 1979
Family Rhizosoleniaceae De Toni 1890
Genus Rhizosolenia Brigtwell 1858
R. bergonii H. Peragallo 1892
R. formosa H. Peragallo 1888
R. hyalina Ostenfeld in Ostenfeld and Schmidt 1901
R. setigera Brigtwell 1858
>
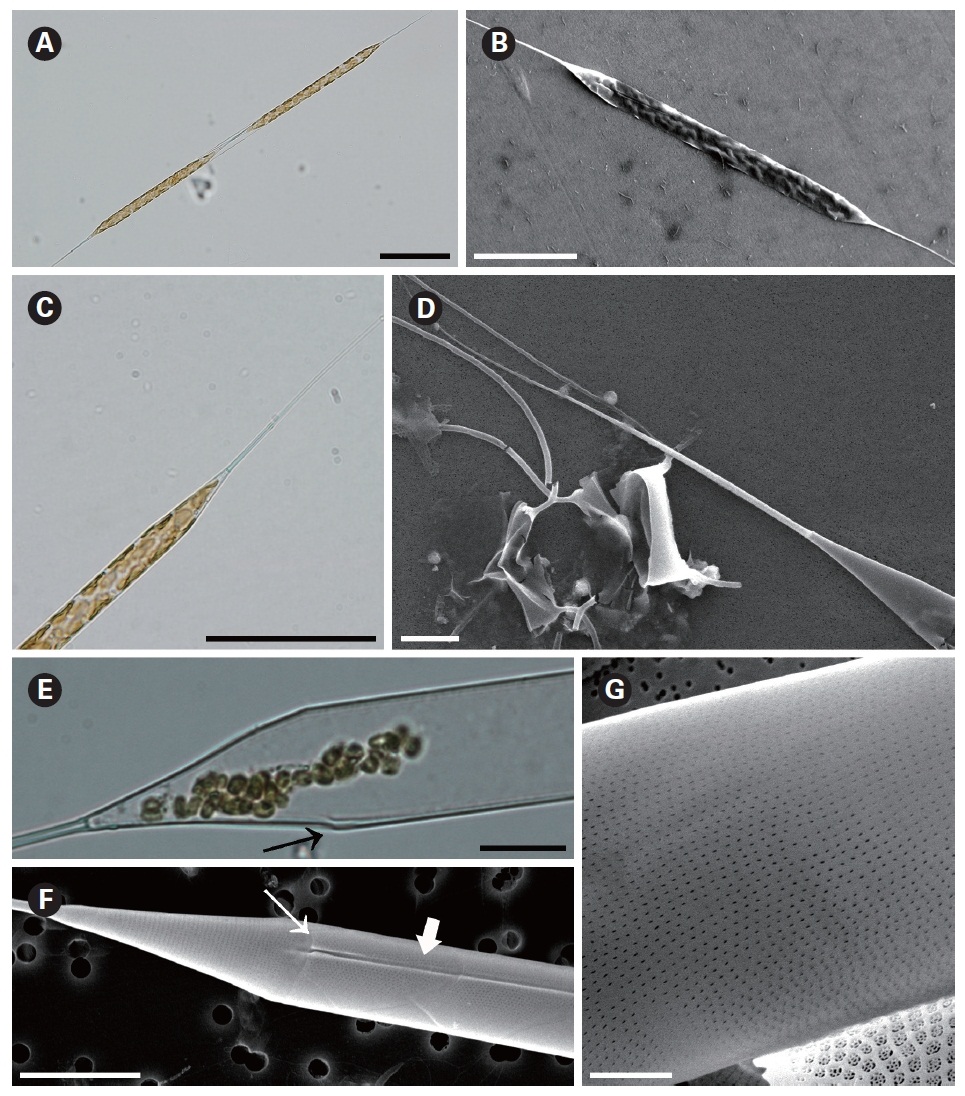
Rhizosolenia bergonii H. Peragallo 1892 (
Peragallo 1892, p. 15, Pl. 3, Fig. 5; Hustedt 1920, p. 318, Fig. 1-3, as
Synonym:
The cells of
[Table 2.] Morphological characters of Rhizosolenia species examined in this study
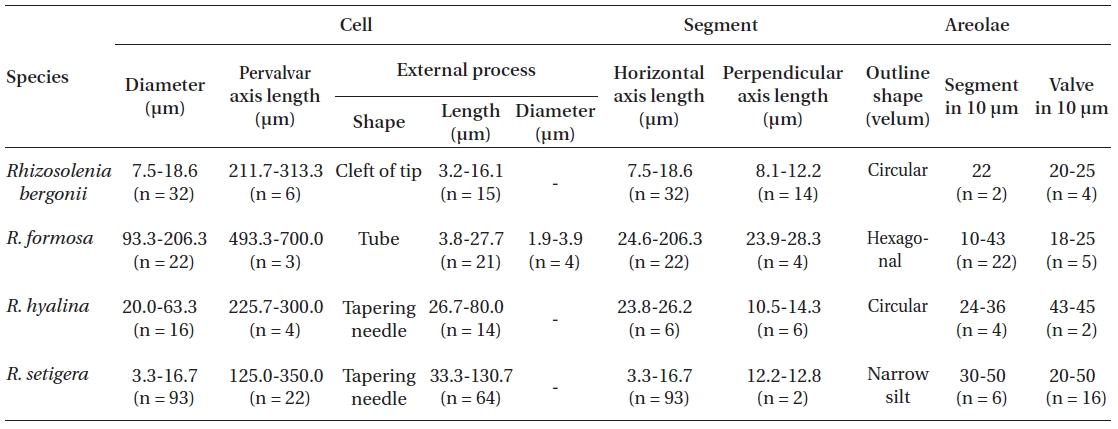
Morphological characters of Rhizosolenia species examined in this study
Distribution:
Remarks:
>
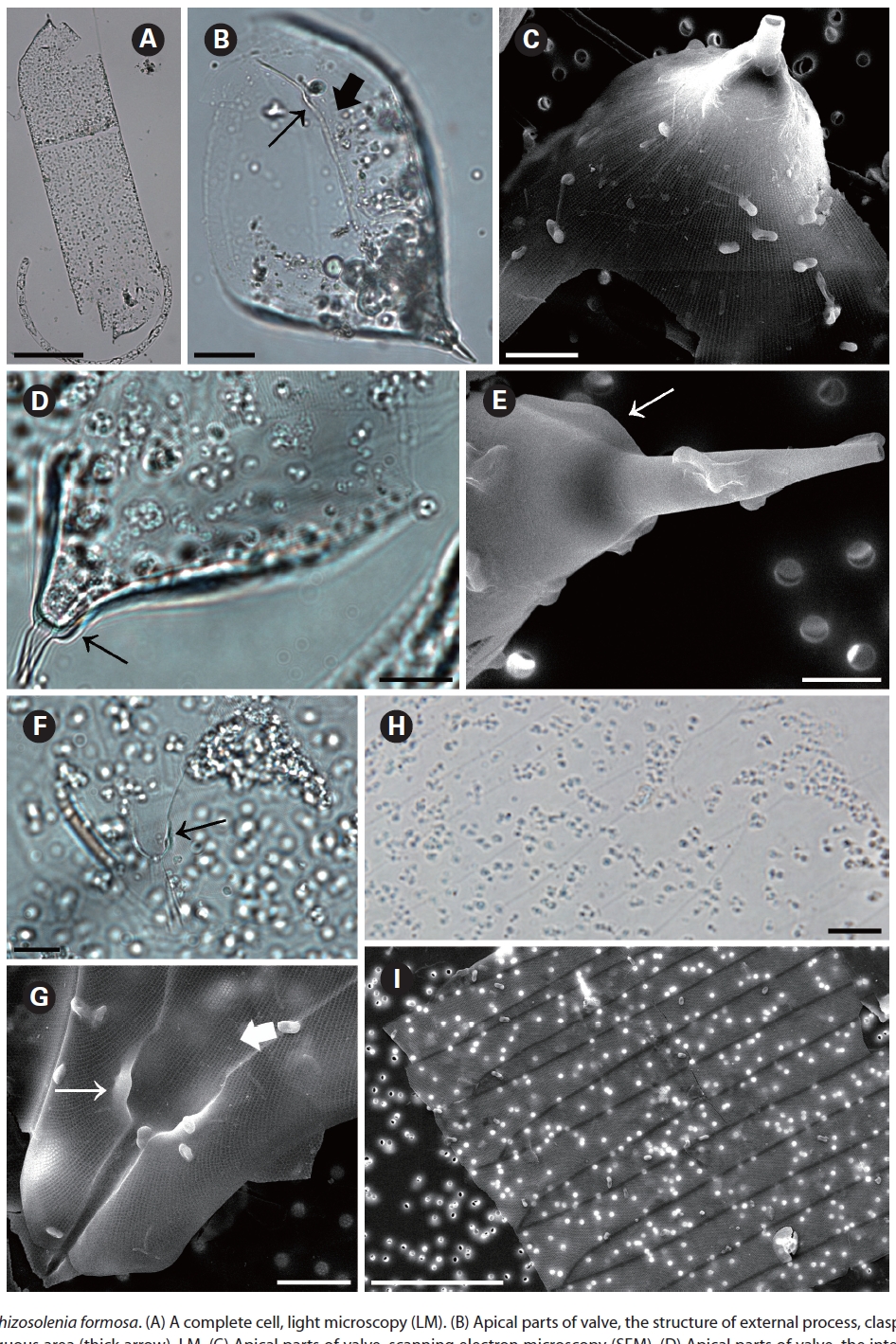
Rhizosolenia formosa H. Peragallo 1888 (
Peragallo 1888, p. 83, Pl. 6, Fig. 43; Sundstrom 1986. p. 33, Fig. 12, 13, 88-93 & 96-99; Hernandez-Becerril 1995, p. 256, Fig. 25-30.
Synonym:
The cells of
two dorsiventral columns, usually of wing shape, median margins short, lateral margins very long. Segment horizontal axis and perpendicular axis are 93.3-206.3 ㎛ and 23.9-28.3 ㎛ length, respectively. Segments areolae, 10-43 per 10 ㎛, with a secondary quincuncial pattern.
Distribution:
Remarks:
>
Rhizosolenia hyalina Ostenfeld in Ostenfeld and Schmidt 1901 (
Ostenfeld and Schmidt 1901, p. 160,Fig. 11; Hustedt 1920, Pl. 319, Fig. 11-13; Sundstrom 1986, p. 76, Fig. 34, 190-194; Hernandez-Becerril 1995, p. 258, Fig. 32-35; Hasle and Syvertsen 1996, p. 151, Pl. 28; Sunesen and Sar
2007, p. 631, Fig. 16-24.
Synonym:
The cells of
Distribution:
Remarks:
>
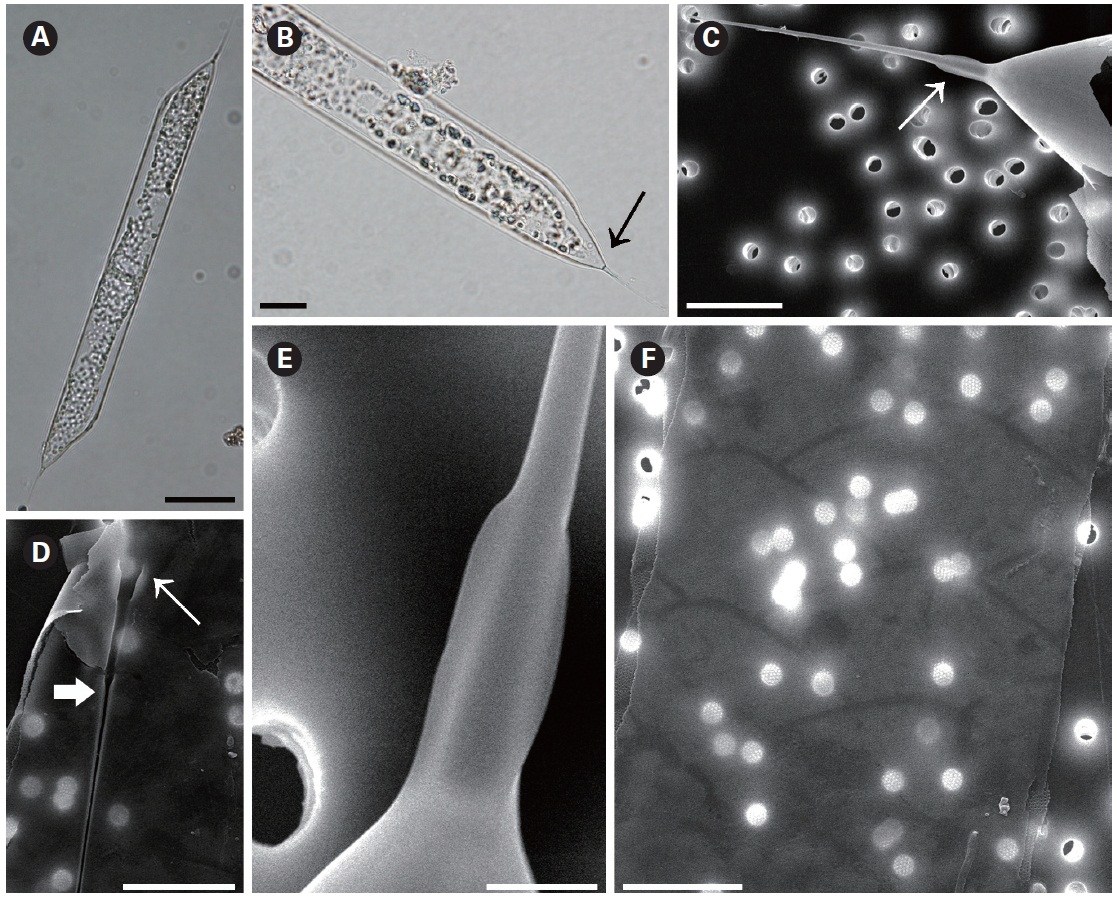
Rhizosolenia setigera Brightwell 1858 (
Hustedt 1930, p. 588, Fig. 336; Navarro 1981, p. 430, Fig. 46 & 47; Sundstrom 1986, p. 104, Fig. 286-288; Hernandez-Becerril 1995, p. 264, Fig. 44 & 45; Hasle and Syvertsen 1996, p. 157, Pl. 30; Sunesen and Sar 2007, p. 633, Fig. 25-34.
Synonym:
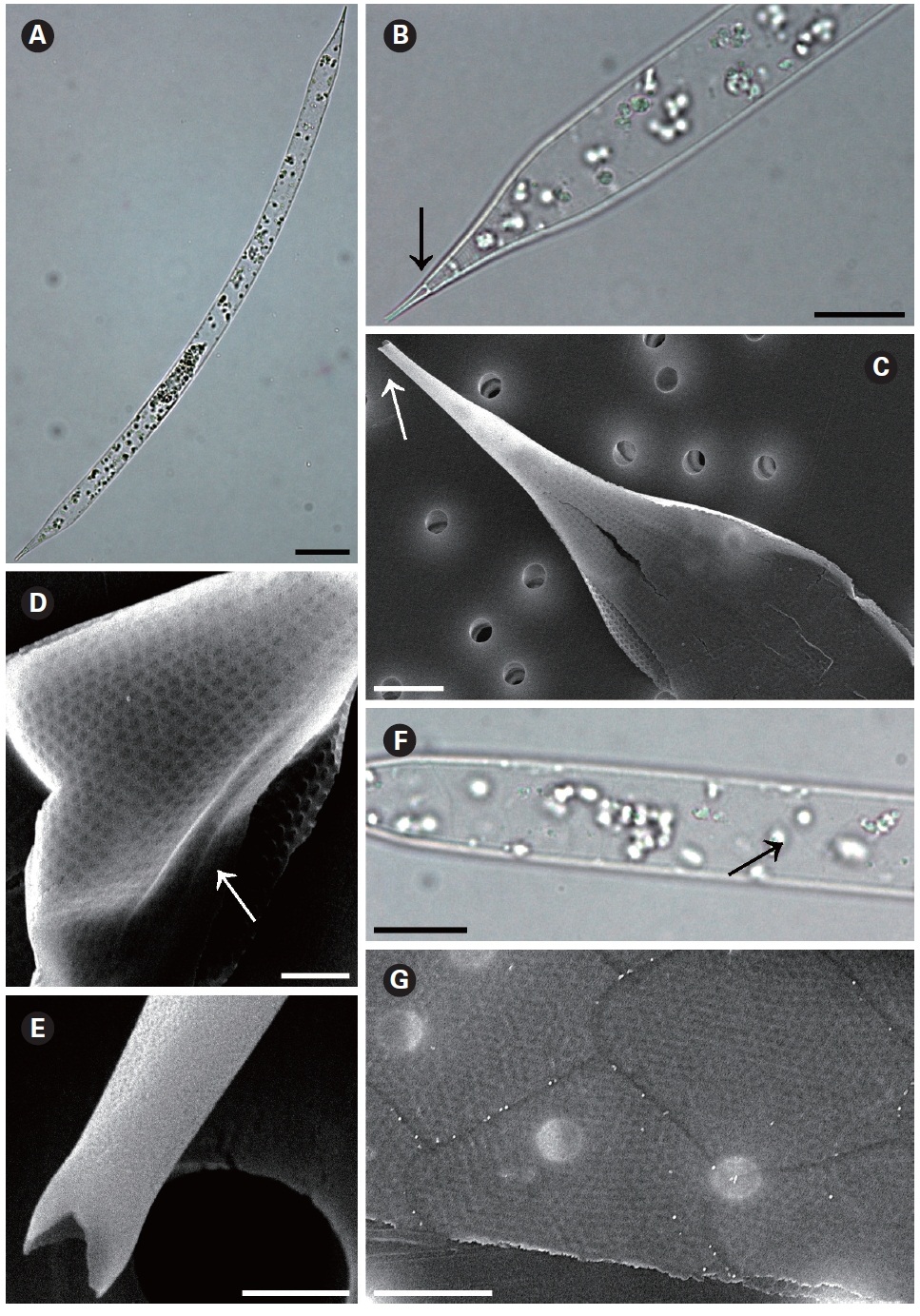
The cells are solitary or in pairs, cylindrical, bilaterally symmetrical, circular in cross-section, 3.3-16.7 ㎛ in diameter, and 125-350 ㎛ long. The valve is sharply sub-conical, elongated. Valve areolae are poroid; Fig. 4. areolae with slit-like pores, 20-50 per 10 ㎛, with a secondary orthostic pattern. Scattered pores are circular, irregularly distributed among areolae or taking the place of some areolae, distinguishable in external view. The process is very long needle shape, almost straight, wider at the base and gradually tapering along the apex, 33.3-130.7 ㎛ long. Contiguous area is observed, groove-shaped, deeper at the basal region than at the terminal region. Otaria are absent, claspers are poorly noticeable. Segment with horizontal axis and perpendicular axis are 23.8-26.2 ㎛ and 10.5-14.3 ㎛ in length respectively. Girdle segments are rhomboidal to trapezoidal and arranged in two dorsiventral columns. Segment areolae are rounded, 30-50 per 10 ㎛, arranged in striae oriented in pervalvar direction, with a secondary quincuncial pattern.
Distribution:
Remarks: