Zinova (1981) established the genus Haraldiophyllum based upon Myriogramme bonnemaisonii Kylin(1924), from Atlantic Europe. The Haraldiophyllum type species, H. bonnemaisonii, is characterized by a broad,erect, foliose thallus with a cylindrical stipe, the absence of a midrib and veins, monostromatic and polystromatic blades, and tetrasporangia sori formed upon both sides of the blade (Maggs and Hommersand 1993). To date,seven species of Haraldiophyllum have been recognized worldwide (Guiry and Guiry 2011), as follows: H. bonnemaisonii(Kylin) A. D. Zinova (1981); H. crispatum (J. D.Hooker & Harvey) Lin, Hommersand & Nelson (2007); H.erosum (Harvey) A. J. K. Millar & J. M. Huisman (1996); H. infossum A. J. K. Millar (1994); H. mirabile (Kylin) Zinova(1981); H. nottii (Norris & M. J. Wynne) M. J. Wynne(1983); and H. sinuosum (A. H. S. Lucas) A. J. K. Millar(1990).
The type species H. bonnemaisonii is typically distributed in the Atlantic Ocean (Maggs and Hommersand 1993, Hardy and Guiry 2003, John et al. 2004, Barbara et al. 2005, Lin et al. 2007). The other six species, however,are endemics with limited distributions. H. mirabile and H. nottii live only along the western coast of North America(Wynne 1983), H. sinuosum and H. infossum occur along the eastern coast of Australia and in the southwestern Pacific (Millar 1990, 1994), H. erosum inhabits west-ern Australia (Millar and Huisman 1996), and H. crispatum occurs in Australia and New Zealand (Lin et al. 2007).
In Asia, Nam and Kim (1996) initially identified H.bonnemaisonii based on the specimen collected from Hadong and Kwangyang along the south coast of Korea.They described the Korean specimens as characteristically having monostromatic blades (except for the basal portion), a procarp with one carpogonial branch and two sterile groups, and tetrasporangial sori on both surfaces of the blades (Nam and Kim 1996). They also noted that Korean specimens reached 10-20 cm in height and consisted of flabellate blades on a prominent stipe; however,Korean H. bonnemaisonii exhibits some differences when compared to specimens from the British Isles (Maggs and Hommersand 1993), having deeply cleft and non-dichotomously lobed blades.
Because the marine algal flora of Udo, Jeju Island, Korea remains very poorly documented, we have collected extensively there to improve our knowledge of red algal biodiversity. The red algae within the Udo marine forests consist of a turf community growing around and between digitate Ecklonia cava holdfasts. While collecting algae from Udo, we found a potentially undescribed species of Haraldiophyllum that belonged to the tribe Myriogrammeae.A careful examination revealed that this alga represented a new species in the Delesseriaceae. Here, we report on this newly discovered species based on its vegetative morphology and rbcL gene sequences, and discuss its taxonomic status among the species of the genus.To elucidate the phylogenetic relationships of the taxa from Udo, Jeju Island, we downloaded the rbcL sequence data of four Haraldiophyllum species from GenBank.
For our morphological study, we collected the specimen on a scuba dive, and preserved them in 4% formalin/ seawater, with the exception of a small piece of thallus to be used for molecular study. The voucher specimen was deposited in the herbarium of Jeju National University(JNUB), Jeju, Korea. We sectioned the preserved specimen by hand or using a freezing microtome NK-101-II (Nippon Optical Works Co. Ltd., Tokyo, Japan).The sectioned preparations were stained with 1% aniline blue acidified with 1% HCl and mounted in 25-30% Karo syrup. To photograph the specimen, we used a Digital Sight DS-Fi1 camera (Nikon, Tokyo, Japan) attached to a microscope (ECLIPSE 80i; Nikon) and then imported all images into PhotoShop 7.0.1 (Adobe Systems, San Jose,CA, USA) for plate assembly.
We air-dried the piece of cleaned thallus and desiccated it using silica gel for the DNA extraction. Using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), and following the manufacturer’s instructions, we ground the silica gel-dried thallus in liquid nitrogen and extracted the total genomic DNA. Extracts were dissolved in 200 mL of distilled water. For amplification and sequencing reactions of genes, we employed the specific primer pairs rbcLF7-rbcLR753 and rbcLF645-rbcS start (Lin et al. 2001,Gavio and Fredericq 2002). For all polymerase chain reaction(PCR) amplifications, we used Swift MaxPro thermal cyclers (ESCO, Singapore) with an AccuPower PCR PreMix (Bioneer, Daejeon, Korea). The PCR comprised an initial denaturation step, at 94°C for 4 min, 35 cycles 1 min at 94°C, 1 min at 50°C, 2 min at 72°C, and a final 7 min extension cycle, at 72°C. To purify the PCR products,we used an AccuPrep PCR Purification Kit (Bioneer) and then sequenced them commercially (Macrogen, Seoul,Korea). Both electropherogram outputs from each sample were edited using the program Chromas version 1.45.
Using BioEdit (Hall 1999), we collated the total sequences and then aligned them visually. No alignments posed problems, as we observed no gaps. The outgroups we used were Augophyllum delicatum (AF257400) and Nitophyllum punctatum (AF257402). To confirm the taxonomic position of a potentially undescribed species of Haraldiophyllum from Udo, we conducted a maximum likelihood (ML) analysis, using PAUP 4.0 (Swofford 2002). We determined the best model for the individual data sets using Modeltest 3.4 software (Posada and Crandall 1998). The best model was a general time reversible(GTR) evolutionary model with gamma correction for among-site variation (Γ) and proportion of invariable sites (I). To estimate ML trees, we used a heuristic search with 100 random addition sequence replicates and tree bisection and reconnection (TBR) branch swapping. To test the node stability, we performed bootstrap analyses with 1,000 replicated ML searches, using the same program and settings.
Description.Thalli ellipticus, 22 cm longis et 6 cm latis, consisto singuli aut aliquot laminae affixus per fibrosus hapteron; lamina in brevis cylindricus stipitis, 2-3 cm longis et 1 mm crassus; lamina tenuis, monostromaticis super, tristromaticis in reproductivus area et polystromaticis versus basis, microscopicus vena absens, integer margo et marginalis cellulae divisus oblique; crescens marginalis et diffuses, sine distinctus cullula apicalis; tetrasporangia eferens in ellipticus sori 2 mm longis in medianas factus simultaneous in superficiebus ambabus lamina; tetrasporangia matura factus bistromatica, spherical, tetrahedraliter divisa, ad 80-100 ㎛ diametro.
Thallus elliptical, 22 cm long and 6 cm wide, consisting of one or several blades attached by a fibrous holdfast; blade on a short, cylindrical stipe, 2-3 cm long and 1 mm thick; blade thin, monostromatic above, tristromatic in reproductive areas, and polystromatic toward the base, microscopic veins absent; entire margins and marginal krcells divide obliquely; growth is marginal and diffuse, without distinct apical cells; tetrasporangia occur in elliptical sori 2 mm long on median, formed simultaneously on both surfaces of the blade; mature tetrasporangia forming two layers, spherical, tetrahedrally divided, to 80-100 ㎛ in diameter.
Holotype. JNU-MSK30601HU, tetrasporophyte, collected on June 14, 2009 (Fig. 1A).
Type locality. Haumokdong, Udo, Jeju Island, Korea (33°30´21˝ N, 126°56´09˝ E)
Type locality. Haumokdong, Udo, Jeju Island, Korea (33°30´21˝ N, 126°56´09˝ E)
Korean name. 우도민엷은잎
Distribution. Known to occur on the south coast (Nam and Kim 1996) and at Udo, Jeju Island, in this study.
Habitat. We collected Haraldiophyllum udoensis at 12 m in the sublittoral zone of Udo, Jeju Island, Korea. The thallus grew as an epilithic blade on a stone covered with Lithophyllum sp. in habitats exposed to strong currents. Among the plants collected along the south coast of Ko-rea by Nam and Kim (1996) and by us for this study, tetrasporophytes were found from March to September. Nam and Kim (1996) collected male and female gametophytes in July and September, respectively, but we collected no gametophytes for this study.
Etymology. We have chosen the specific epithet to represent the name of the islet collection site-Udo, Jeju Island.
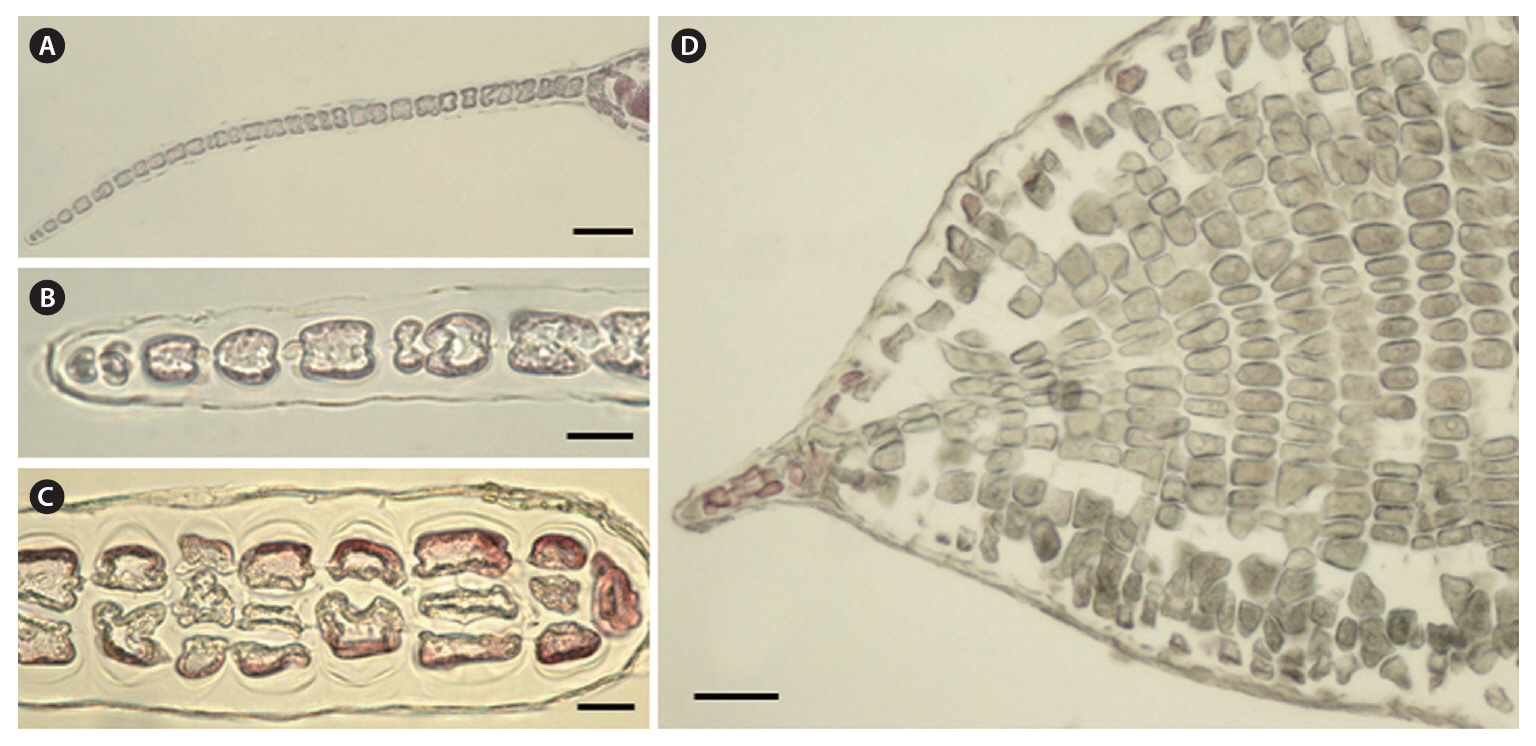
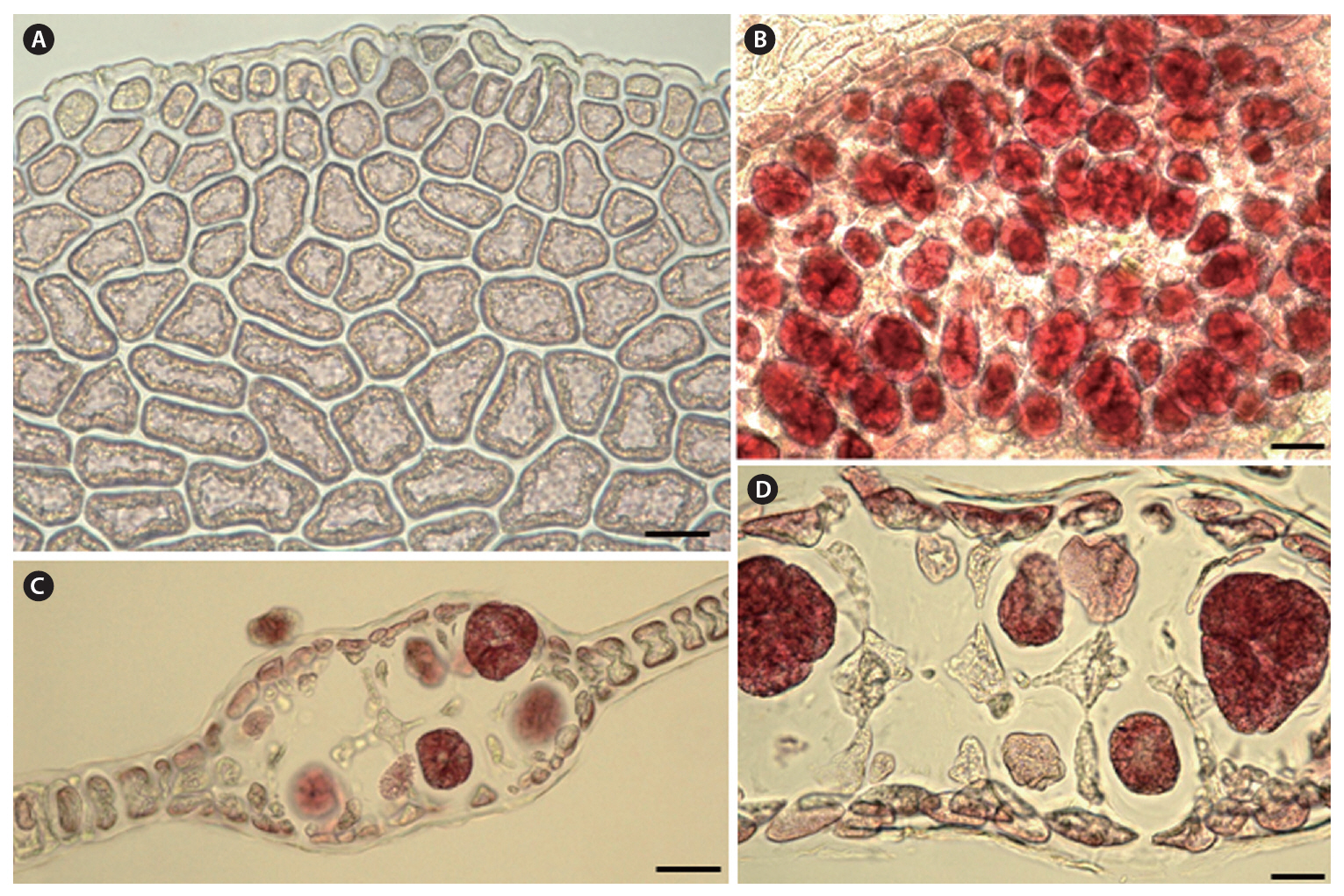
Morphology.The plant is a greenish red-brown, 22 cm high, 6 cm broad, foliose, and erect on a cylindrical stipe up to 2-3 cm long and 1 mm thick (Fig. 1A). The thallus consists of one or several blades, attached by a small fibrous holdfast, that lack midrib and microscopic veins (Fig. 1B & C). The blade is thin and monostromatic above, becoming tristromatic in reproductive areas and polystromatic toward the base with evanescent midribs (Fig. 2A-D), and increasing in thickness from 35 ㎛ near the apices to 100 ㎛ basally. The blade comprises cells that are elongate-polygonal in surface view (Fig. 3A) and 45-70 ㎛ long × 15-45 ㎛ wide, linked by secondary pit connections that increase in abundance in the polystromatic regions. The blade has entire margins, and marginal cells divide anticlinally or obliquely, growing marginal meristems without distinct apical cells (Fig. 3A).
Tetrasporangia are produced in small, numerous, circular to elliptical sori, formed randomly on both sides of the blade, and scattered throughout the blade except along the margins and the lowermost parts. Tetrasporangial sori are 2 mm long × 0.6 mm wide, and protrude on either side of the blade, approximately 200 ㎛ thick (Fig. 3B). In the transverse section of the sori, the tetrasporangia appear in two layers between both surface cells (Fig. 3C). The tetrasporangia are produced from inner cortical cells but occasionally also from primary cells. Mature tetrasporangia are spherical, 80-100 ㎛ in diameter, and divided tetrahedrally (Fig. 3D).
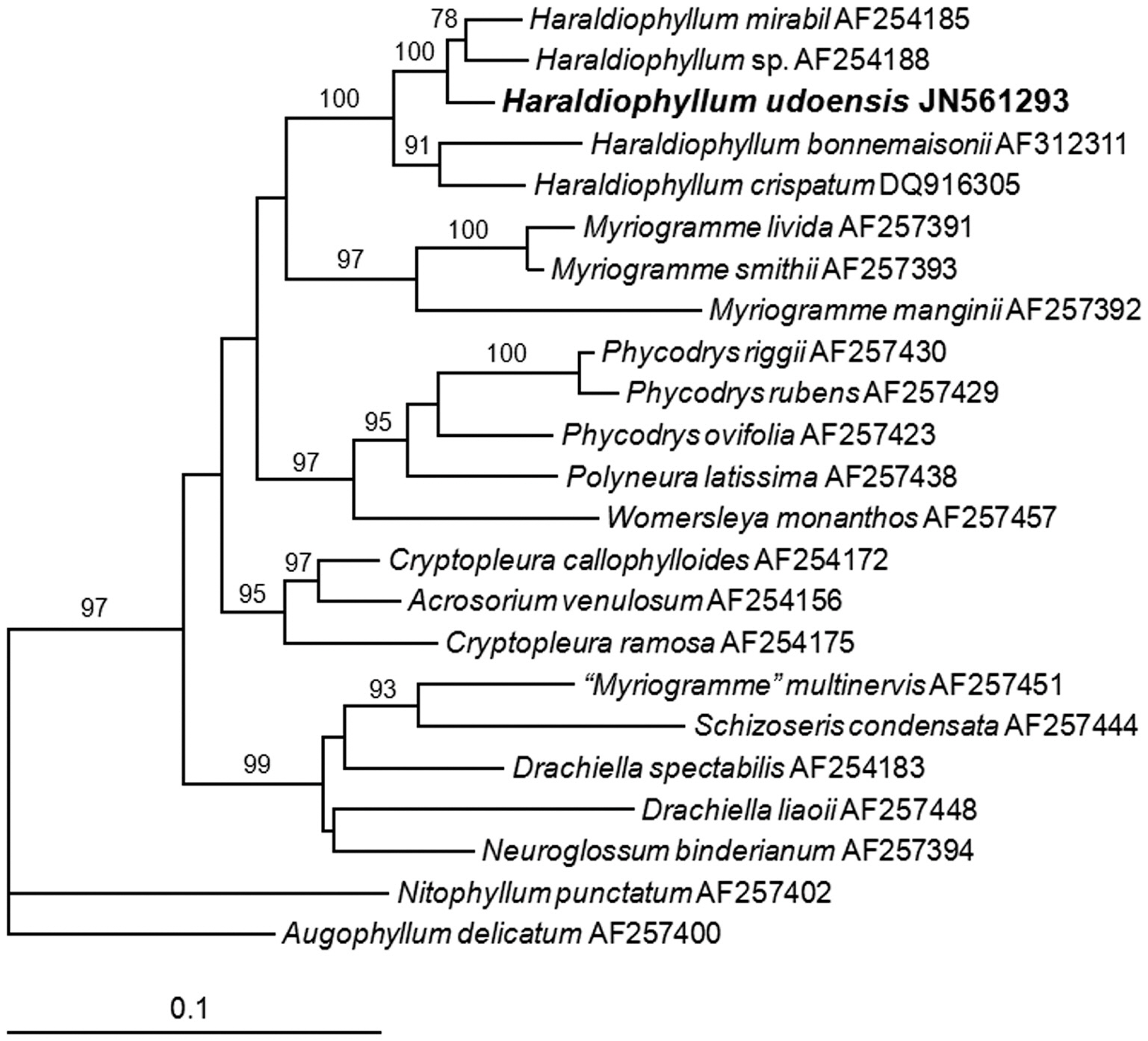
Molecular analysis of rbcL.To align the five Haraldiophyllum species with putative relative genera in the family Delesseriaceae, we used a 1,303 nucleotide (nt) portion of rbcL gene and two outgroups. Variable sites occurred at 464 positions (35.6%), and 350 positions (26.9%) were parsimoniously informative. In the ML analysis, we estimated the ?ln likelihood score as 7,899.6647 under the GTR + Г + I model. The sequences differed by up to a 72 bp (5.5%) pairwise distance between H. udoensis from Korea and H. bonnemaisonii from the United Kingdom. The new species differed from H. crispatum from New Zealand, H. mirabile from the United States, and Haraldiophyllum sp. from Chile by 64, 32, and 39 bp, respectively. The uncorrected sequence divergence (in terspecific p distance) values for the rbcL region within Haraldiophyllum ranged from 31 bp (2.7%) between H. mirabile from the United States and Haraldiophyllum sp. from Chile to 87 bp (6.7%) between H. bonnemaisonii and Haraldiophyllum sp. from Chile. The most-related genus, Myriogramme, differed by 109-142 bp (8.3-11%) from H. udoensis and by 103-169 bp (8-13%) from the rest of the genera we tested in the present study.
In the phylogenetic tree from rbcL, the new species formed an independent clade with strong support (99% for ML) and a sister group with H. mirabile from the United States and Haraldiophyllum sp. from Chile (Fig. 4). The ML analysis of rbcL data indicated that H. udoensis showed clear separation from H. bonnemaisonii and H. crispatum from New Zealand. The ML tree showed two groups within Haraldiophyllum, and the bootstrap supports were quite strong (100% and 91%). The first clade included H. udoensis as the new species, H. mirabile and Haraldiophyllum sp., and the second clade contained H. crispatum and H. bonnemaisonii.The genus Haraldiophyllum monophyly had definitive bootstrap support (100%). The genus Myriogramme formed a sister relationship with the genus Haraldiophyllum.
Haraldiophyllum udoensis is newly described from Udo, Jeju Island, Korea, based on our detailed morphological observations and molecular analysis. This new species is characterized by the following features: 1) one or several elliptical blades on a short, cylindrical stipe with fibrous roots; 2) monostromatic blades, except at the base and on reproductive structures; 3) a lack of network and microscopic veins; 4) entire margins with a lack of proliferations; 5) growth by means of many marginal initials; and 6) an arrangement of two distinct tetrasporangia layers. Molecular phylogenetic analysis based on rbcL sequences demonstrated H. udoensis’s distinctness from its congeners, and separated it clearly from the European H. bonnemaisonii. The genus Haraldiophyllum contains two clusters of species, one from the United Kingdom and New Zealand and the other occurring along the Pacific Coasts of Korea, the United States, and Chile. Our molecular analysis produced similar results to those Lin et al. (2007) reported. The new species is epilithic in the sublittoral zone at depths up to 12 m. It was previously identified in Korea as H. bonnemaisonii (Nam and Kim 1996). Although H. udoensis closely resembles the type species of Haraldiophyllum (H. bonnemaisonii from the
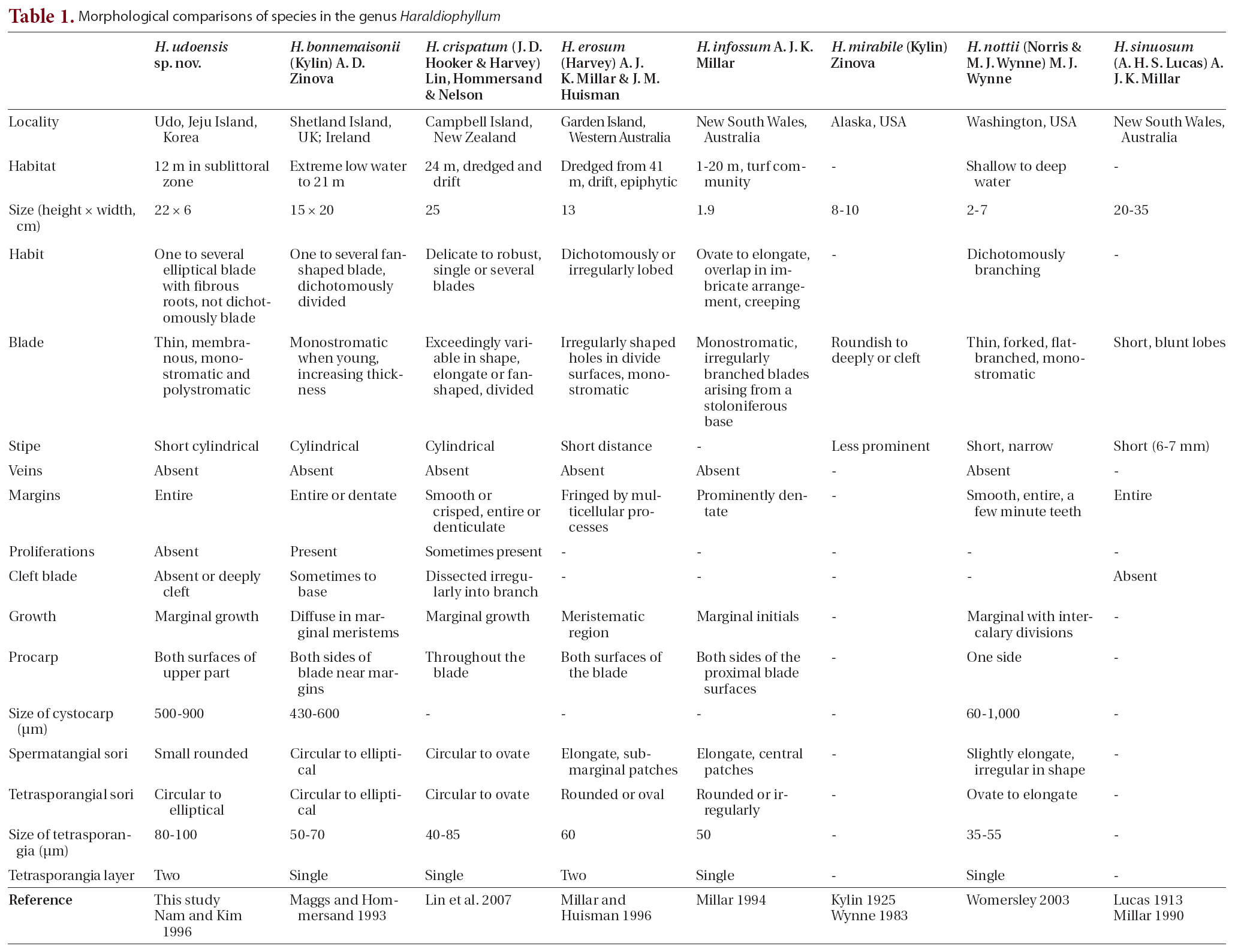
British Isles), H. bonnemaisonii has a distinct thallus that divides regularly and dichotomously into overlapping lobes, frequently proliferating on the blade and forming a single tetrasporangia layer in a zigzag manner (Maggs and Hommersand 1993, Lin et al. 2007). In addition, the reproductive structure in H. udoensis is larger; the cystocarp diameter of > 700 ㎛ contrasts with the < 600 ㎛ in H. bonnemaisonii, and tetrasporangia in H. udoensis are > 80 ㎛ in diameter, as opposed to < 70 ㎛ in H. bonnemaisonii (Table 1).
In recognizing the species of Haraldiophyllum, which currently contains seven species, the vegetative characteristics are viewed as important diagnostic features, such as whether thallus size is diminutive, whether shape of the base and margins are dentate or entire, whether the branching pattern is dichotomous, whether the lobed blade is deeply cleft, and the presence or absence of stipes (Table 1). Thallus size is useful for identifying species of Haraldiophyllum; H. bonnemaisonii and H. crispatum reach 20-25 cm, whereas H. infossum and H. nottii are 2-7 cm smaller than other species (Millar 1994, Womersley 2003, Lin et al. 2007). In particular, H. infossum from Australia differs from all other species in the genus due to its diminutive size (up to 1.9 cm). Haraldiophyllum sinuosum is by far the largest of the genus with plants reaching lengths of 20-35 cm. Haraldiophyllum erosum and H. mirabile are of medium size at around 10 cm (Millar and Huisman 1996), but the new species H. udoensis is approximately 20 cm in length. The shapes of the base and margins have been demonstrated as valid characteristics for delineating some species of Haraldiophyllum, as for example Millar and Huisman (1996) showed changes of the Haraldiophyllum species’ margins display a continuum from species with entire margins to those with fringed margins. Most of Haraldiophyllum species including the new species H. udoensis have entire or dentate margins, except for two: H. erosum and H. infossum (Millar 1994). According to Millar and Huisman (1996), H. erosum from western Australia has an unusual marginal fringe with numerous multicellular
processes. They report these marginal processes are often polysiphonous and divaricately branched, and they always terminate single apical cell in a transversely-dividing (Millar and Huisman 1996). H. infossum often has a stoloniferous base and prominently dentate blade margins (Millar 1994).
The branching pattern in relation to the presence or absence of lobed blades and stipes can also be an important distinguishing feature. H. bonnemaisonii comprises one to several fan-shaped blades, borne on cartilaginous stipes and dividing dichotomously into overlapping lobes with rounded apices (Maggs and Hommersand 1993, Lin et al. 2007). H. crispatum has an exceedingly variable shape, dividing partially into broad lobes with rounded margins or cleaving into irregular, dichotomously-branched segments with proliferations (Lin et al. 2007). H. erosum’s blades have dichotomous or irregular lobes and often have irregularly-shaped holes in the surfaces (Millar and Huisman 1996). H. infossum has ovated to elongated individual blades that often overlap in an imbricate arrangement with the creeping stoloniferous parts compressed (Millar 1994). H. mirabile from Alaska has less prominent stipes, and the blades are roundish to deeply lobed or cleft (Kylin 1925). The blades of H. nottii are complicated and subdichotomously branched with lobed, narrow stipes (Norris and Wynne 1968). The blades of H. sinuosum are generally entire and not deeply cleft but have short, blunt lobes, and the plants have short stipes that do not persist into the lower blades (Lucas 1913). The new species H. udoensis appears to differ from other species mostly by its one to several elliptical, non-lobed blades.
Researchers have documented the diagnostic characteristics of the genus Haraldiophyllum’s reproductive structures for the female reproductive organs, such as the procarp, carposporangia, and cystocarp. The procarps contain two groups of sterile cells, one group of cover cells and two periaxial cells parallel to the frond’s longitudinal axis (Millar 1994). The carposporangia are borne singly and terminally on gonimoblast filaments. The cystocarp has a fusion cell incorporating gametophytic cells at the floor of the cystocarpic cavity. Kylin (1925) noted this feature of the cystocarp fusion cell is probably useful for generic recognition of the genus Haraldiophyllum. Maggs and Hommersand (1993) and Millar (1994) also included this characteristics in their generic circumscription of Haraldiophyllum, while Millar and Huisman (1996) recognized two different sequences of cell formation leading to the procarp’s final structure in Haraldiophyllum. They observed H. bonnemaisonii has only one sterile cell group cutting off from the supporting cell prior to the initial carpogonial branch. The production of the second sterile cell group delays until after carpogonial branch formation. The same sequence occurs in H. crispatum, H. erosum, and H. nottii, but that of H. infossum differs in forming the two sterile cell groups before initiating the carpogonial branch (Millar 1994). Millar and Huisman (1996), therefore, concluded these differences appear to be stable within the Haraldiophyllum species. In the new species, H. udoensis, procarp formation and cystocarp development follow a pattern similar to those previous studies describe (Millar 1994, Millar and Huisman 1996, Nam and Kim 1996, Lin et al. 2007).
While researchers often use the female reproductive organ’s structure to distinguish species within the genus and genera in the family, they have not considered the number of layers in the tetrasporangia arrangement to be of any significance in this genus. The type species H. bonnemaisonii has only a single layer of tetrasporangia arranged in a zigzag manner, and the same feature occurs in H. crispatum, H. infossum, and H. nottii. However, two species, H. udoensis sp. nov. and H. erosum, form two layers of tetrasporangia. To verify the importance of the number of layers in the tetrasporangia arrangement from a phylogenetic perspective, further observation and molecular analysis for all seven species are necessary.
The genus Haraldiophyllum superficially resembles two genera, Myriogramme and Nitophyllum, although Haraldiophyllum clearly belongs to the tribe Myriogrammeae based on vegetative and reproductive developmental characteristics (Hommersand and Fredericq 1997). The genus Haraldiophyllum is readily separated from the genus Myriogramme by carposporangia organization, which is terminal solitary in Haraldiophyllum but terminal chains in Myriogramme (Wynne 1983, Lin et al. 2007). The new species H. udoensis forms a separate clade from Myriogramme based on molecular analysis of rbcL sequence data. However, the new species is linked to Myriogramme as a sister clade with low bootstrap support in the ML analysis. Haraldiophyllum is distinguished from the genus Nitophyllum by the number of sterile cell groups in the procarps, with two for Haraldiophyllum and only one for Nitophyllum, as well as by the two periaxial cells’ alignment with respect to the frond’s longitudinal axis, showing a parallel manner in Haraldiophyllum and a perpendicular manner in Nitophyllum (Zinova 1981, Wynne 1983). In the molecular phylogenetic tree, the Haraldiophyllum clade is distantly separate from the Nitophyllum clade including its type species, N. punctatum from Spain.
Our ongoing studies of the Delesseriaceae from Jeju Island, Korea have thus far clarified the generic placement of Haraldiophyllumand led to the recognition of a new species, H. udoensis. On Jeju Island, many delesseriaceous taxa remain that require critical reexamination in light of recent taxonomic developments within the family.