One of the remarkable features in the intertidal environment is emersion and submersion of seaweeds by tidal cycles. Intertidal seaweeds commonly experience nutrient limitations as soon as they are exposed to air and suffer from desiccation (Brawley and Johnson 1991, Davison and Pearson 1996). Algal propagules are susceptible to stress and their physiological responses to emergence are clearly related to the subsequent distribution and abundance (Brawley and Johnson 1991, Vadas et al. 1992, Davison et al. 1993). Crowded germlings are able to survive under desiccation conditions that would normally kill well-spaced individuals. This phenomenon has been tested on the shore (Ang and De Wreede 1992, Andrew and Viejo 1998) and in simulated tidal regimes in the laboratory (Hruby and Norton 1979). Crowding negatively affects germling performance under favorable growth conditions whether they are in monoculture or mixed with another species (Reed et al. 1991, Creed et al. 1996, Choi and Norton 2005
It is generally known that upper shore algae, both in the juvenile and adult stage, are better adapted to emersion stresses than the plants inhabiting the lower shore (Chapman 1995, Davison and Pearson 1996). One can reasonably speculates that germlings of the upper shore species may be more tolerant for the stress than those of lower shore algae, but this has not been experimentally tested. It is of particular interest how the physiological responses of germlings differ between species and whether the outcome of competition in mixtures of species is changed by the degree of the stress. There is evidence that the outcome of competition in mixtures of two species in the adult stage has been altered by temperature, irradiance and nutrient availability (Enright 1979, Fujita 1985, Peckol and Rivers 1995). A few interspecific competition studies have been conducted at the germling stages (Choi and Norton 2005
Stress tolerance of seaweeds to emersion stress is also age-dependant (Brawley and Johnson 1991, Davison et al. 1993).
The vertical distribution pattern of fucoid algae is consistent on many rocky shores in northern Europe:
Twenty fertile plants of both
>
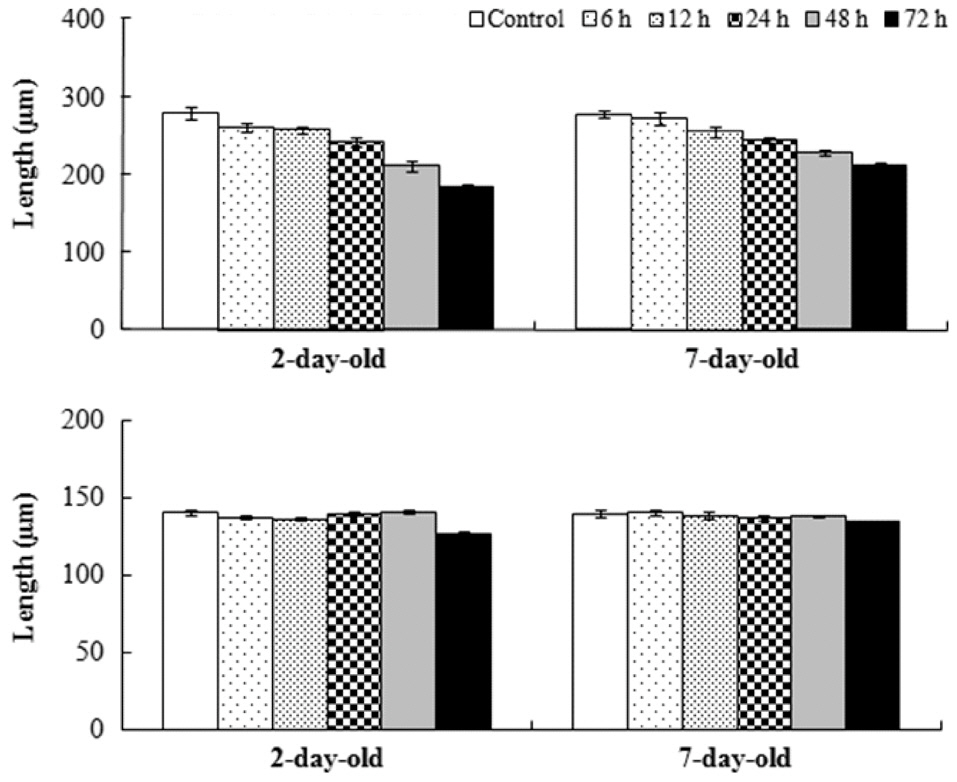
Effects of desiccation on different age germlings
To create uniform settlement density, 5 mL of zygote suspension (60 zygotes mL-1) of each species was inoculated into two Petri dishes for each of six treatments (control, 6, 12, 24, 48, and 72 h). Each dish contained eight glass slides cut to 2.5 × 2.0 cm and 30 mL of autoclaved seawater. After 2 days, 12 slides (from the 16 in each treatment) on which germlings were most evenly distributed were chosen; four were used to determine settlement density, and eight were used for the experiment. For the culture experiment, four replicate dishes were used, each containing six slides, one from each treatment, and 50 mL of culture medium (Kain and Jones 1964). Germlings were cultured at 10 ± 1ºC , 120 ㎛ol photons m-2 s-1 and a photoperiod of 16 h : 8 h LD. The culture medium was changed every 3 days.
The effects of germling age on their mortality and growth were examined after moving the slides with attached germlings from the culture dishes into a desiccation tank (33 × 22 × 21 cm) with a relative humidity of 70-90%. Germlings were moved to the desiccation tank after culturing germligs for 2- or 7-days in the dishes. After the desiccation period (6, 12, 24, 48, and 72 h), the slides were returned into the culture dishes and cultured for 16 days. Control slides were continuously cultured in culture dishes.
Settlement density was determined by counting the number of settled germlings within four 4 mm2 areas on each slide. Settlement density of germlings was ca. 30 germlings cm-2. Frond lengths of 25 germlings were measured for each species and each slide. Germling mortality was estimated by counting 100 germlings on each slide after 16 days.
>
Effects of density and periodic submergence in a tidal tank
Twenty fertile plants of
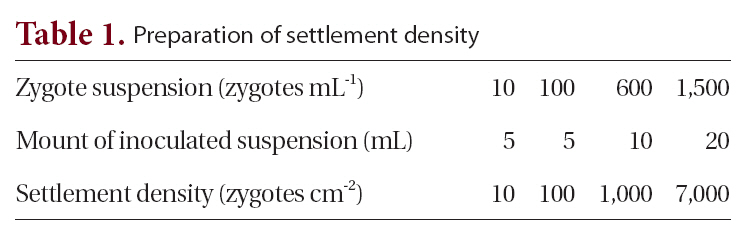
[Table 1.] Preparation of settlement density
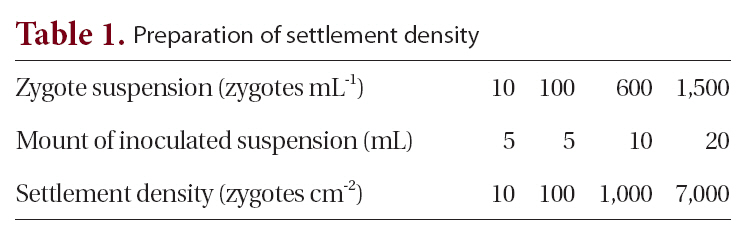
Preparation of settlement density
After 2 days, 20 slides (from the 24 in each density treatment) on which germlings were most evenly distributed were chosen: four were used for determining density and 16 for the experiment. For the culture experiment, four replicate tidal tanks were used, each containing 16 slides, four from each treatment. The four slides were placed on four steps each exposed to air for different periods (none, 6, 8, and 10 h / 12 h tidal cycle) in 4 tidal tanks see below. Thus, each step had twelve glass-slides (4 density × 3 proportion levels) as described in Fig. 1. Culture conditions were 10 ± 1ºC, 16 h : 8 h LD, 120 ㎛ol photons m-2 s-1 and the culture medium was not changed for a period of 16 days.
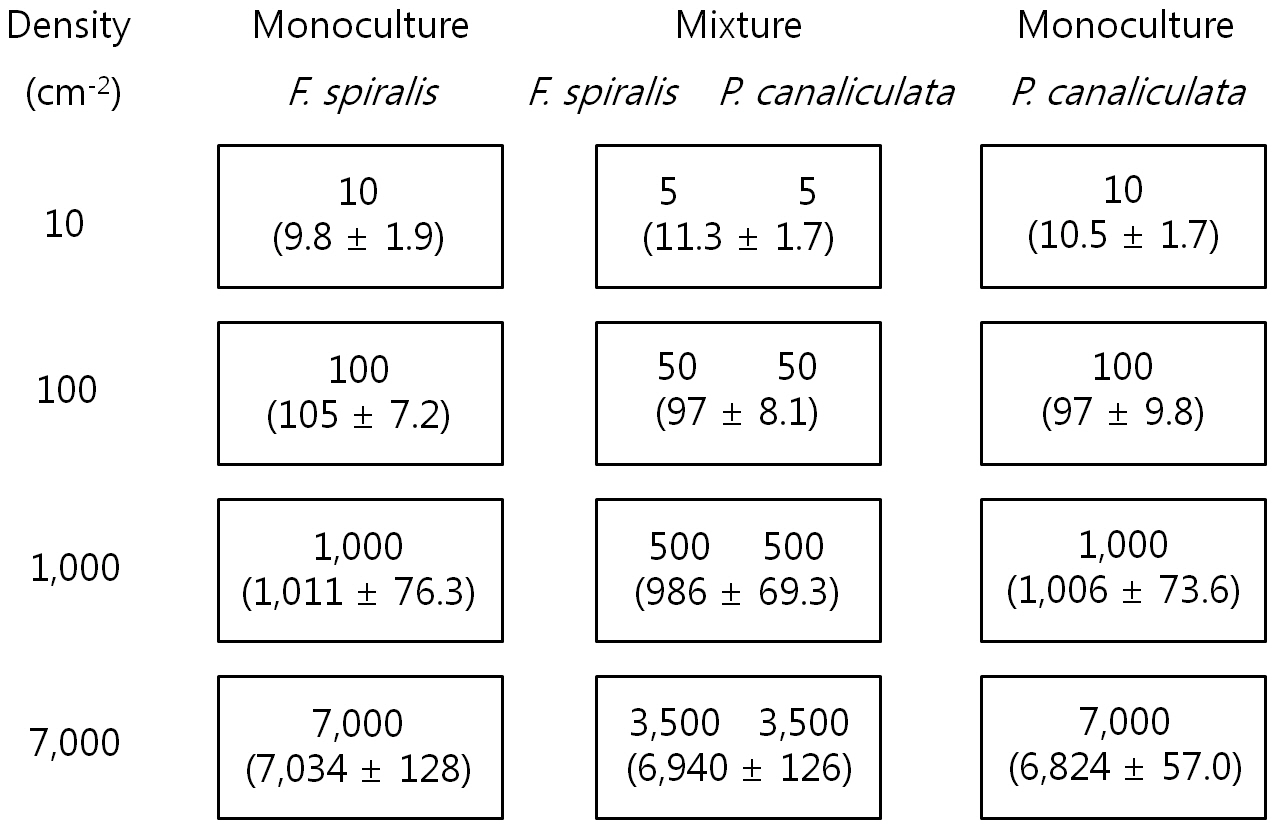
Settlement density of germlings was determined after setting up the experiment and the lengths and mortalities of germlings were measured after 16 days as described above. The mean settlement density of four replicates is shown in Fig. 1. There were significant differences in density (analysis of variance [ANOVA], p ? 0.05), but not between proportions of each species within a density.
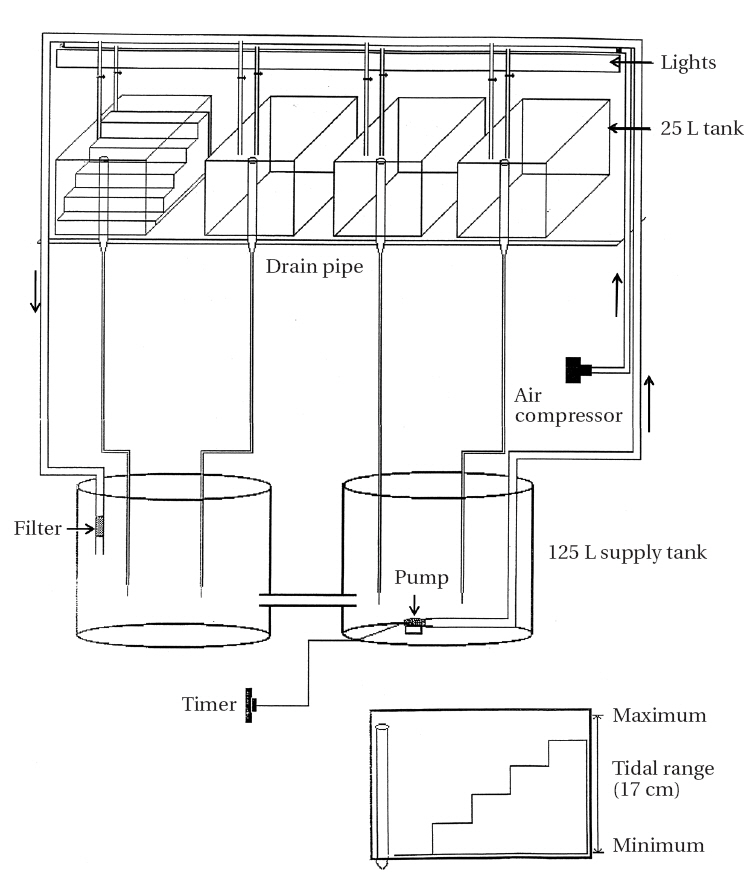
Artificial tidal tanks (25 L) were prepared for periodic submergence experiment (Fig. 2). Four tanks were placed on a shelf in a culture-room and connected with two tanks each containing 125 L of culture medium. An aquarium pump (Aquaclear Power Head 400; Hagen, Germany) was placed in the reservoir tank from which seawater levels of the tidal tanks were gradually increased when the pump operated for 6 h twice per day (6-12 AM and 6-12 PM). An automatic timer was used to control water pump operation. Water level decreased gradually when the pump stopped and water drained out continuously at a rate of
60 mL per minute through outlet pipes (Fig. 2). Thus, two tidal cycles per day were simulated in the tidal tanks.
A perspex “staircase” was placed on the bottom of each tank. The lowest of the five steps was submerged continuously and the duration of air exposure increased by increasing the step height (none, 4, 6, 8, and 10 h / 12 h tidal cycle). Relative humidity of tidal tanks was 70-90%.
Data were analyzed using one-way and two-way ANOVA. Homogeneity of variances was tested by Cochran’s test. Where necessary, data were transformed before analysis to meet the assumptions of parametric tests (Sokal and Rohlf 1995). The significance of the differences between means was tested with the Tukey HSD test.
>
Effects of desiccation on different age germlings
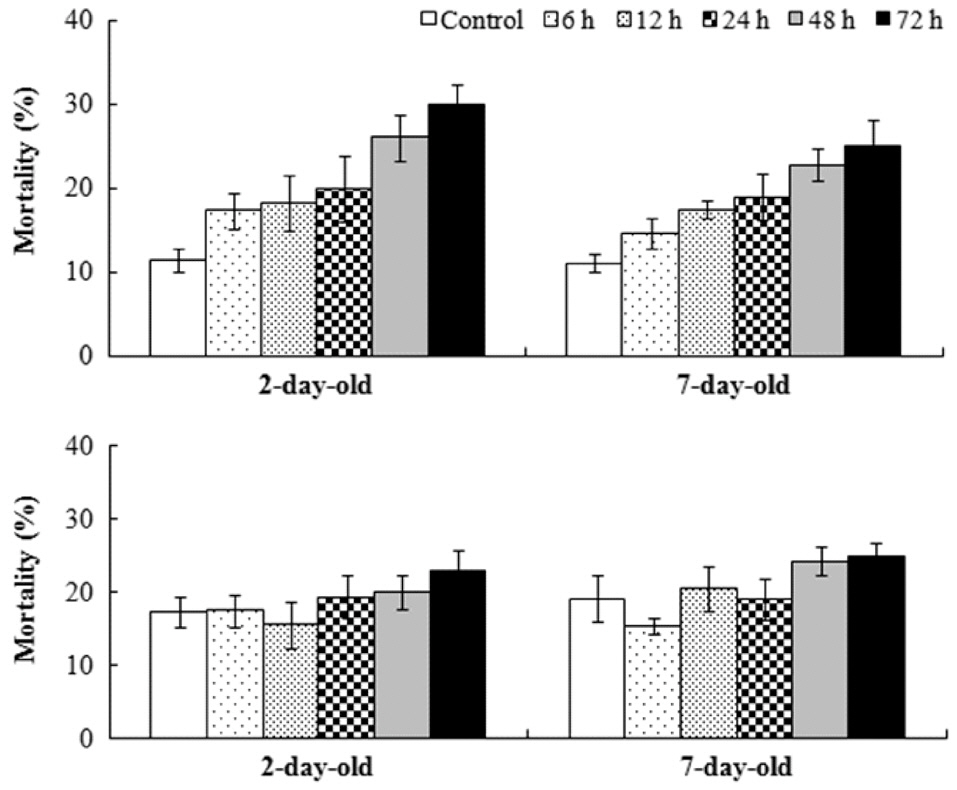
Sixteen days after settlement, the mortality of
Zygote diameter was 106.66 ± 11.28 μm (mean ± standard error, n = 60) for
Mean relative growth rate of
The relative growth rate was significantly greater when germlings were exposed to air after 7 days rather than after 2 days in
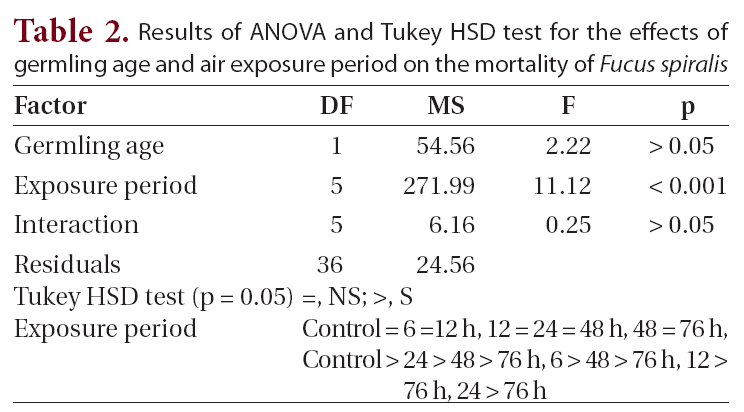
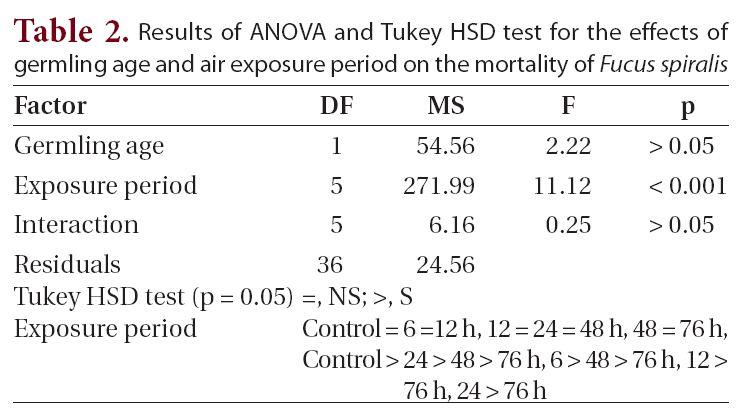
Results of ANOVA and Tukey HSD test for the effects of germling age and air exposure period on the mortality of Fucus spiralis
were found among the other treatments. Significant differences for
>
Effects of density and periodic submergence in a tidal tank
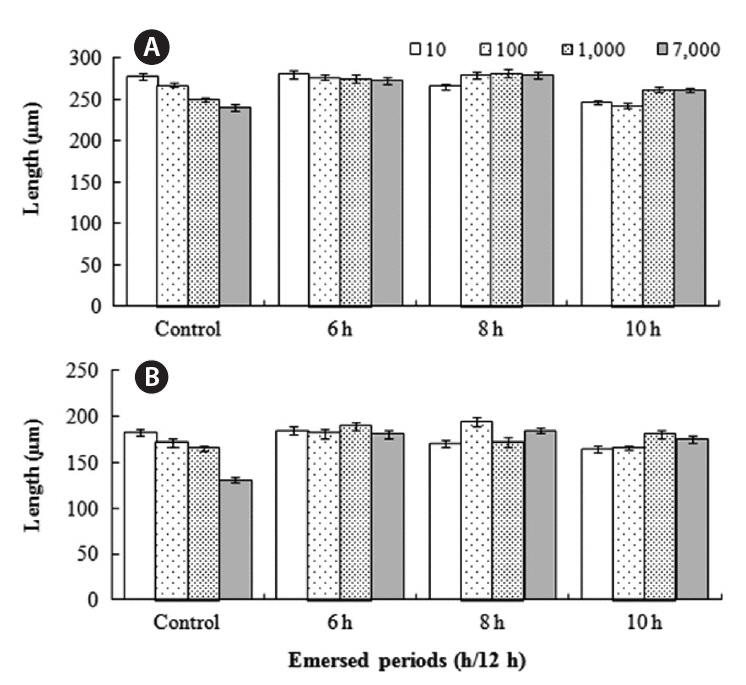
Germling mortality was not significantly affected by the density of germlings or by the periodic exposure periods of the two species 16 days after settlement. Mortality was 8.3-15.6% for
In monoculture,
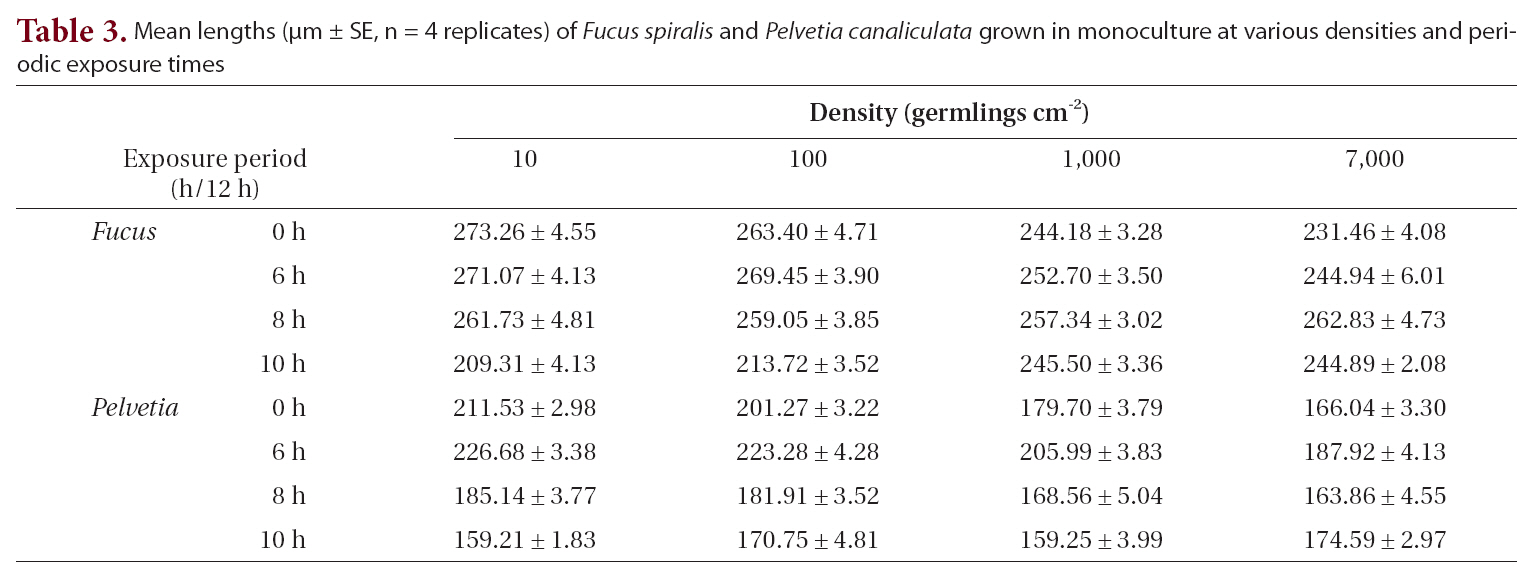
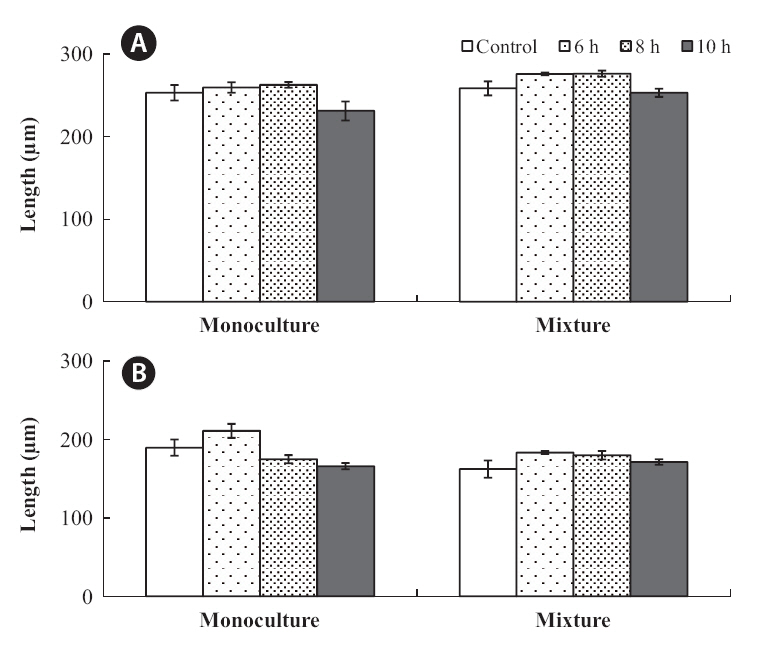
The growth of both species in monoculture was influenced by germling density and by periodic exposure period. After 16 days in culture, the mean lengths of germlings were 209-273 μm for
In the mixtures, germling lengths were 240-282 μm for
for 6 or 8 h compared to the control and 10 h treatments, and no significant difference was observed between the 6 and 8 h or between the control and 10 h treatments (Tukey test). Growth was significantly greater when
The density and growth relationship changed from negative to positive by increasing the duration of emergence (Fig. 5), resulting in no statistically significant influence of density on
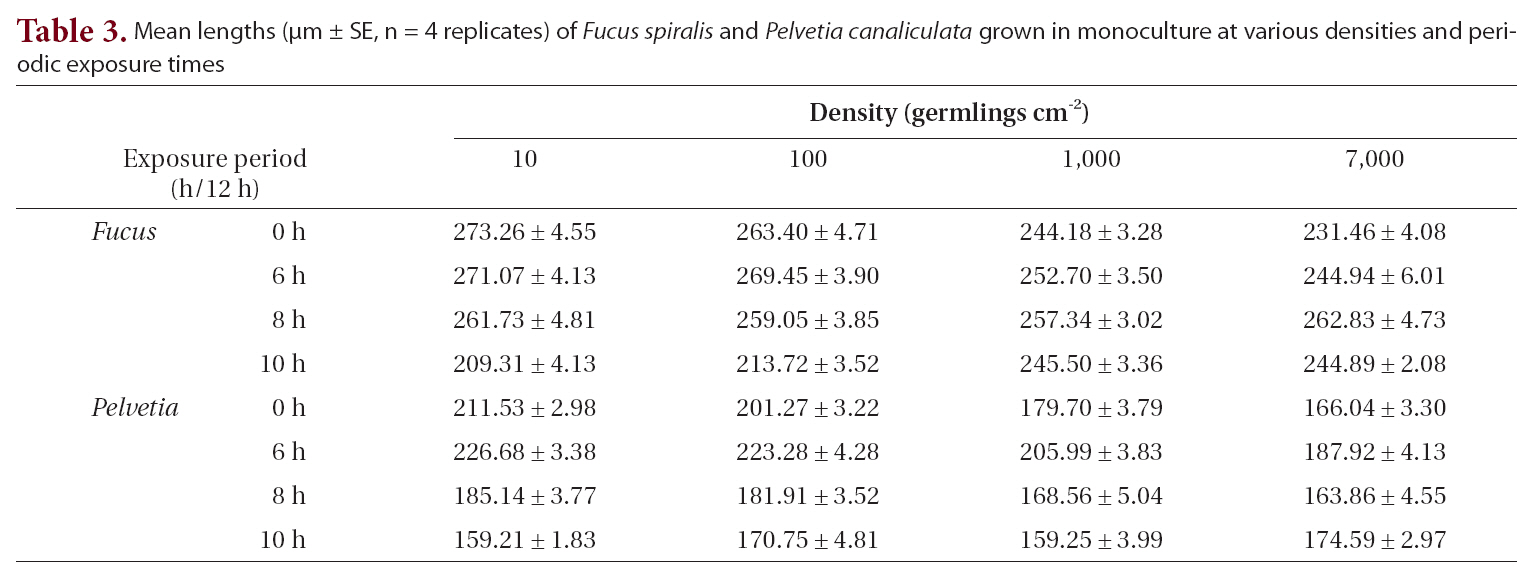
Mean lengths (μm ± SE n = 4 replicates) of Fucus spiralis and Pelvetia canaliculata grown in monoculture at various densities and periodic exposure times
The relative importance of intra- and interspecific competition was compared with respect to the growth of both species under the various emerged periods (Fig. 6). The mean lengths of the four densities within each treatment were pooled for the comparisons.
Over the range of density levels, germling growth was also significantly affected by duration of emergence for
Emersion stress inhibits photosynthesis and retards embryonic development, and the ability of
Under emerged stress, crowded germlings survive well by mutual protection compared to germlings that are sparsely settled (Hruby and Norton 1979). The present results show that the survival of
The outcome of interspecific competition between