Using chlorophyll fluorescence, the vertical migration of benthic diatoms responding to light intensity and affected by sediment grain size was studied. Minimal fluorescence (Fo) of surface sediment was measured by imaging pulse amplitude modulated (Imaging-PAM) fluorometer, and used to monitor diatom biomass variation in surface sediments. The test diatoms, Amphora coffeaeformis (C. Agardh) Kutzing and Cylindrotheca closterium (Ehrenberg) Reimann & Lewin,migrated to the sediment surface under irradiance from 50 to 500 ㎛ol photons m-2 s-1. However, the diatoms exhibited no evident increase of surface biomass under dark conditions, and even showed slightly decrease of surface biomass under irradiances over 1,000 ㎛ol photons m-2 s-1. The light intensity inducing the maximum surface migration of A.coffeaeformis was 100 ㎛ol photons m-2 s-1, while the light intensity producing the same effect for C. closterium was 250㎛ol photons m-2 s-1. C. closterium showed higher motility than A. coffeaeformis. Faster diatom surfacing was observed in larger grain size sediments (125-335 ㎛) than smaller ones (63-125 ㎛). This study confirmed the significant influence of light as a main triggering factor behind migration, indicated the distinct effect of different sediment grain size,and highlighted the species-specific migratory ability.
Benthic diatoms inhabiting intertidal sediments exhibit vertical migratory rhythms within the upper sediment layers, which are associated with diurnal and tidal cycles (Round and Palmer 1966, Palmer and Round 1967,Joint et al. 1982). This phenomenon is particularly well documented for estuarine intertidal microphytobenthos(MPB)(Guarini et al. 2000, Consalvey et al. 2004). Since vertical migration of microphytobenthos has been largely recognized as a key controlling factor of short-term variability in microphytobenthic productivity, it has been studied increasingly in recent years (Pinckney and Zingmark 1991, Serodio et al. 2001).
It has been considered that the main reasons behind this vertical migration are endogenous phototaxis and geotaxis, primarily in response to light and tide (Harper 1977, Consalvey et al. 2004). Furthermore, the diel cycle of light is the main factor triggering MPB migration in subtidal zones with nearly no influence of tidal cycles (Ni Longphuirt et al. 2006). Due to the large variation of light intensity under
Some environmental factors, such as temperature and salinity, affect MPB motility (Paterson 1986, Cohn and Disparti 1994, Sauer et al. 2002, Cohn et al. 2003). Besides,sediments grain size is an important factor that is closely associated with light penetration, porosity, water content, and dissolved nutrients, as well as MPB biomass and species composition (Underwood and Kromkamp 1999, Mitbavkar and Anil 2002, Bale and Kenny 2005). This factor is also thought to influence the speed or depth to which diatom cells migrate (Hay et al. 1993, Consalvey et al. 2004). Previous studies have proven that speeds of diatom movement are different on various substrata, and vertical speeds are an order of magnitude lower than horizontal speeds (Hopkins 1963, Harper 1977, Hay et al. 1993). Migratory speed of diatoms is comparatively slower in sediments than on artificial substrata, such as glass slides. It has been observed in the field that the diatoms concentrated at a depth of 1 mm can migrate up to the surface in 1.5 hours (Hopkins 1963). Sediments components are different in grain size. Therefore, knowing the effect of grain size on migration is helpful for analyzing motility of diatoms in sediments, and for elucidating the distinction of species composition in different sediments.
Various techniques have been utilized to investigate vertical migration, including direct observation of color change (Aleem 1950, Perkins 1960), the lens tissue technique(Eaton and Moss 1966), and cryofixation for low-temperature scanning electron microscopy (Paterson 1986, Janssen et al. 1999). In recent decade, the techniques utilizing spectral reflectance and fluorescence have been widely used to monitor changes in microphytobenthos biomass (Serodio et al. 1997, Kromkamp et al. 1998, Paterson et al. 1998, Perkins et al. 2001, Honeywill et al. 2002). Serodio et al. (1997) initially employed fluorescence techniques that use a pulse amplitude modulated(PAM) fluorometer to monitor biomass of benthic microalgae. It showed that minimum fluorescence (
Until now, no study has used Imaging-PAM to investigate vertical migratory behavior of benthic diatoms, even though several laboratory studies have examined this behavior by focusing on the influence of light, temperature,and salinity. In this study, we use Imaging-PAM to monitor surface biomass variation of thin layer sediment,which covers the artificial diatom biofilm in the wells of 24-well plates.
We aim to investigate the effects of light and sediment grain size on vertical migration of individual diatom species, as well as analyze the migration mechanism through physiological and morphological characteristics of diatoms with a miniaturized experimental setup.
Diatoms
The sediment, which was collected from the sand flats of Nakdong River estuary, was treated to remove organic materials. Firstly, sediment was sifted to remove shell fragments and gravel. Then, it was rinsed several times with tap water to remove most of the salt. Subsequently, 33% H2O2 was added to the sediment. It was mixed and left for several days to allow for the complete reaction to remove organic matters (Taylor et al. 2005). After decanting the overlying water, sediment was rinsed with distilled water at least 10 times, and then with deionized water at least 5 more times. Finally, sediment was dried at 60oC in a dry oven for 8 h. Certain sediment of specific grain sizes (63-125, 125-250 and 250-335 ㎛) was obtained by serially dry-sieving.
>
Sediment chlorophyll a concentration and minimal fluorescence (Fo)
This experiment determined variation of
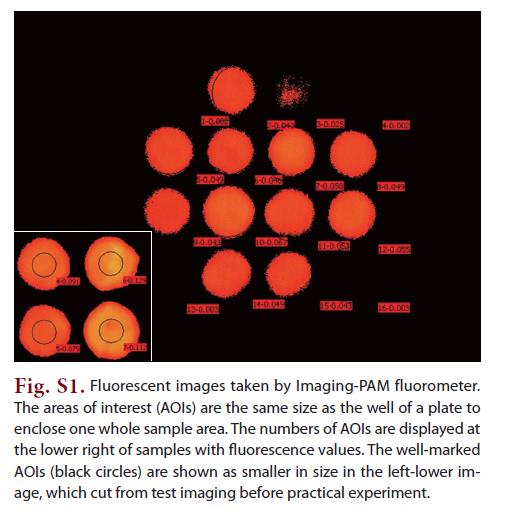
During every measurement using the Imaging-PAM fluorometer (Max/L, Walz, Germany), the well plate was put on a fixed mounting stand position under the measuring head of the Imaging-PAM. Before measuring fluorescence,areas of interest (AOIs) were defined under Live Video Mode, with the same size as a plate well. The same AOIs were consistently used in one set of the experiment. After a 5 min dark adaptation,
After measuring fluorescence, the chlorophyll a of sediments over filter paper was extracted in 90% acetone at 4oC under dark conditions. Chlorophyll
>
Experiments on vertical migratory photoresponse
Preparation of the well plate was the same as previously described, viz. adjusting sample height in wells with 3.0 g treated sediments (125-250 ㎛). Cultured diatoms were deposited homogenously on glass microfiber filters (porosity 2.3 ㎛) by slow filtration (< 0.1 MPa), and then covered with approximately 1 mm thick sediments. The
In every experiment set, initial
The first set of experiments focused on the effect of light intensities on the vertical migratory response. Three replicates of each species were treated with 7 light intensities of 0, 50, 100, 250, 500, 1,000, 1,400 ㎛ol photons m-2 s-1, respectively. Covered sediments used 125-250 ㎛ grain size sand. Samples were incubated at 20oC.
The second set of experiments focused on the effect of sediment grain size on the upward vertical migration. Three kinds of grain size sediment (63-125, 125-250 and 250-335 ㎛) were used to cover the artificial biofilm of three replicates. Samples were incubated at 20oC and 100 ㎛ol photons m-2 s-1 for up to 2 h.
Univariate analyses, followed by post-hoc Tukey tests, were carried out to test the difference between different light intensities and different grain size sediment using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
>
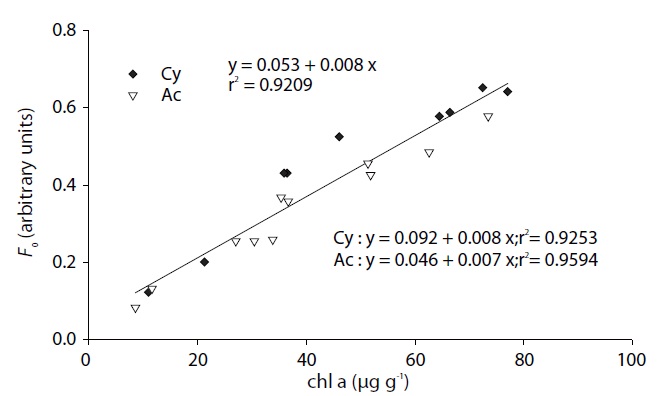
Correlation of chlorophyll a concentration and Fo
Sediment chlorophyll
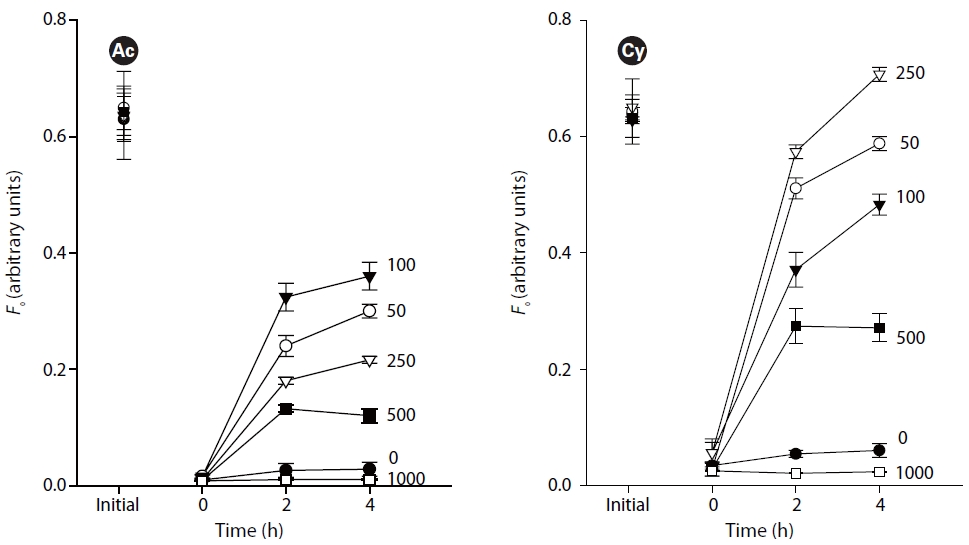
Fig. 2 illustrates the migratory responses of individual species to different light intensities. The lowest
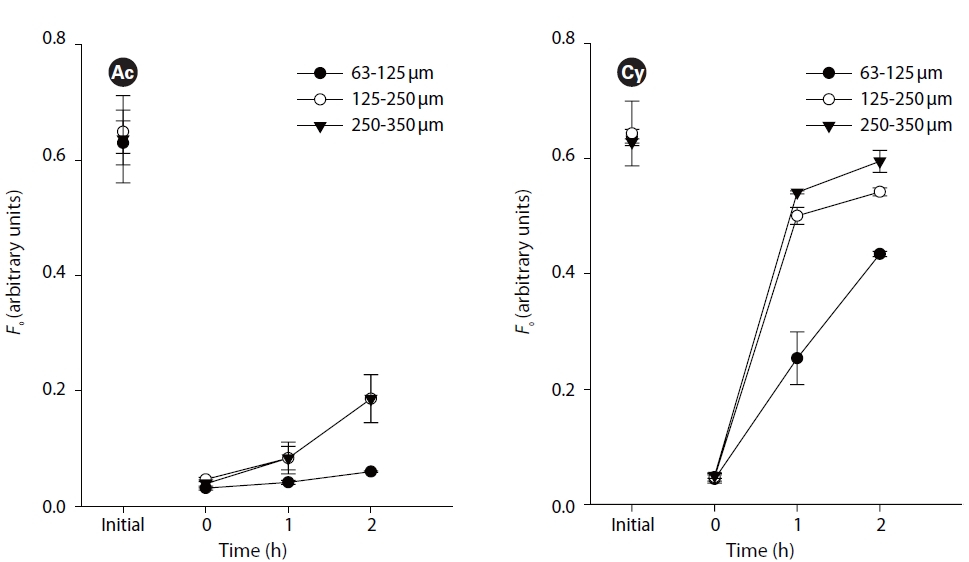
Small (63-125 ㎛) grain size showed significant difference from medium (125-250 ㎛) and large (250-335 ㎛) grain size (p < 0.05, Fig. 3). However, there was no difference between middle and large grain sizes (p > 0.05, Fig. 3). Comparing similar initial values,
>
Validity of laboratory studies
The designed experimental setting in this study was proven valid, practical and convenient for studying vertical migratory behaviors of benthic diatoms. Despite an unavoidable oversimplification of natural variability, a laboratory-based investigation was still appropriate for studying the effects of some main environmental factors on vertical migration. Under the experimental conditions in this study, it was evident that cultured diatoms showed their phototaxis by moving towards the light. The stratified structure used in the experimental design was representative for benthic biofilms
>
Monitoring benthic diatoms biomass through minimal fluorescence (Fo)
By measuring minimum fluorescence
A dark adaptation period of 15 min has been suggested to measure
>
Light effect on vertical migration
The study revealed obvious phototaxis of diatoms under low to moderate irradiances (50-500 ㎛ol photons m-2 s-1). Under dark conditions, without light, there were no evident surfacing movements. High light intensity over 1,000 ㎛ol photons m-2 s-1 kept the diatoms out of the very surface of sediments where
Vertical migratory response to different irradiances has also been observed on intact biofilms of estuarine sediments (Serodio et al. 2006), namely that surface biomass increases under irradiances below 100 ㎛ol photons m-2 s-1, reaches maximum under 100-250 ㎛ol photons m-2 s-1, and gradually decreases under higher irradiances.
>
Grain size effect on vertical migration
Light penetration depth in sediment is closely related with sediment characteristics. Penetration depth in reconstituted and intact sediment was 2-3 mm at most, and deeper in larger size sediment under higher light intensity (MacIntyre et al. 1996). Sediment porosity (i.e., the space that diatoms move through between the grains) is closely related to sediment compaction and grain size (Flemming and Delafontaine 2000). However, the influence of sediment fabric, bulk density, and porosity on the speed of diatom locomotion through sediments has remained unclear until now.
This study is the first attempt to determine the effect of sediment grain size on vertical migration of different species. The obvious difference between small grain size sediment and medium and large grain size sediment is the effect on the upwards migratory photoresponse. It confirmes that sediment characteristic are important factors in influencing the diatoms migration. One reason is that diatom cells can easily sense stronger light stimuli,inducing upward migration in sediment with larger grain size. Another important reason is that larger size sediment grains supply a larger space and shorter traveling distance for the movement of diatom cells. In other words, under experimental conditions without disturbance by hydrodynamic forces, grain size effect on diatom migration is mostly related to its physical property of porosity and surface area.
>
Species-specific migratory response and ability
In this study, migratory response to light was different between two species. The diatom
These species-specific responses have their origin in physiological characteristics. Round and Palmer (1966) observed that
Besides, higher motility of