The natural growth of a population of Gomphosphaeria aponina Kutzing (Chroococcales, Cyanoprocaryota) was studied in a cemented freshwater tank in Allahabad, India. This population appeared to be a polymorphic species. Different species of the genus Gomphosphaeria have been segregated based on morphological features of colonies, cells and mucilage. However, these features are not well defined for different species. Our observations revealed many feature variations and, interestingly, certain features that have been described for different Gomphosphaeria species were seen in a single population. In this study, records of such variable morphological features were possible due to the availability of numerous specimens and continuous observations for more than two years. Further, this study revealed two points: (i) more detailed morphological studies are required both from nature as well as in culture to identify critical differences among the species, and (ii) molecular characterization of taxa appears to be necessary for final species settlement.
Current taxonomic criteria of Cyanobacteria include polyphasic data from morphology, ecology, biochemistry and molecular studies. However, most researchers have to rely on morphological features described in classical literature, so long as complete information remains unavailable for taxa identification. Classical information is mostly based on single collections and is limited in the information that early researchers could initially develop. We need to study the morphology of individual taxa or populations in more detail and, of course, without ignoring data from other sources. Komarek (2005) suggested that morphological and ecological descriptions of Cyanoprocaryotes are as important as their molecular studies. Even if molecular studies are considered the base line for tracing the evolutionary series, the value of serious morphological studies are important and useful in understanding structure and function relationships of the organisms.
This study is concerned with the coccoid genus,
A natural population of Gomphosphaeria was found growing in a small cement tank (8' W × 12' L × 5' D) in Allahabad, India. Growth of the organism was kept under observation for more than two years (March 2007-December 2009). Microscopic observations were made with a Leica DMLB microscope with a DC 300 camera (Leica Microsystems, Plymouth, MN, USA). All photo-micrographs were taken of fresh specimens collected from the cement tank, mounted in water without any staining. During the study of
Occurrence: We found the colonies of
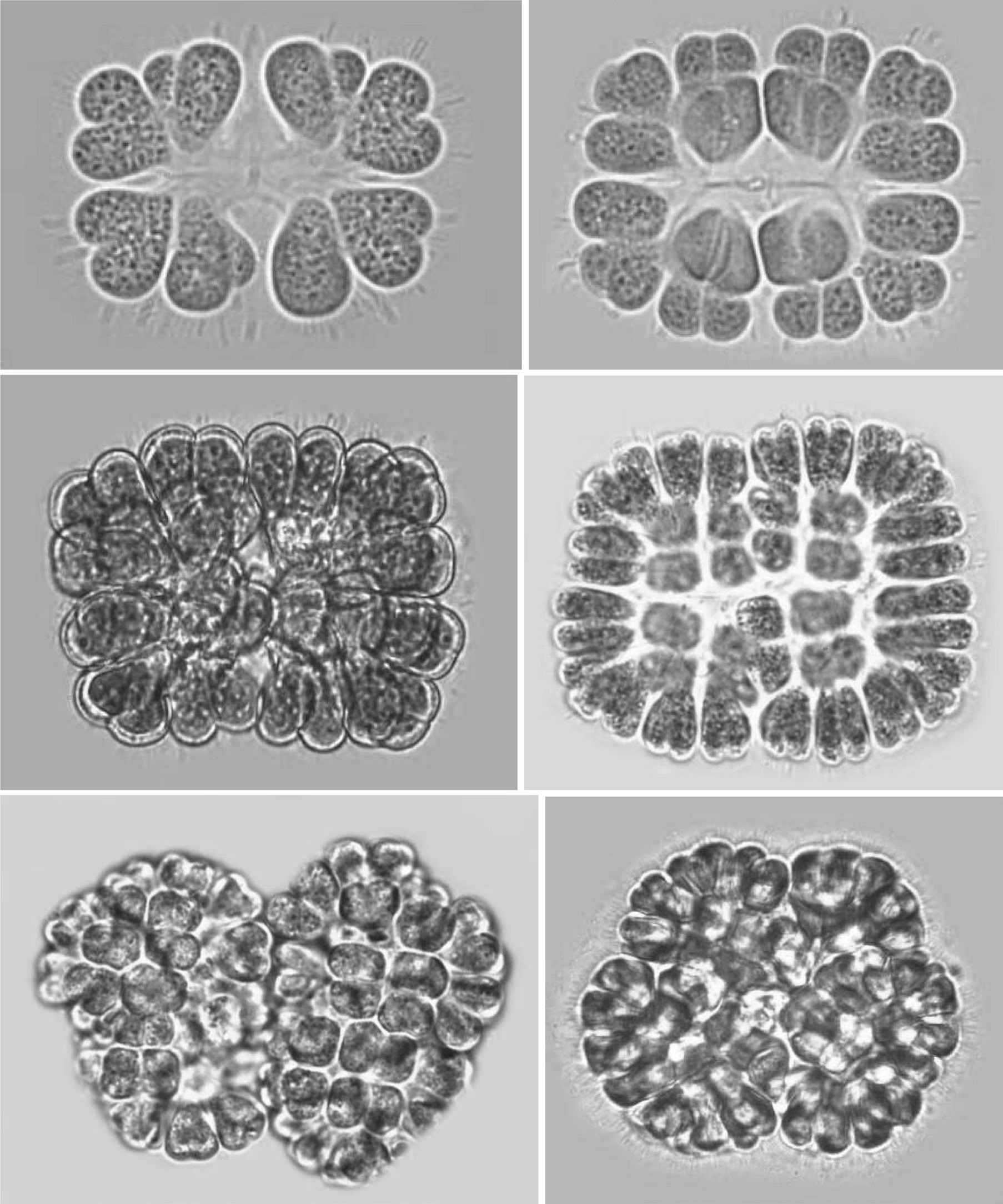
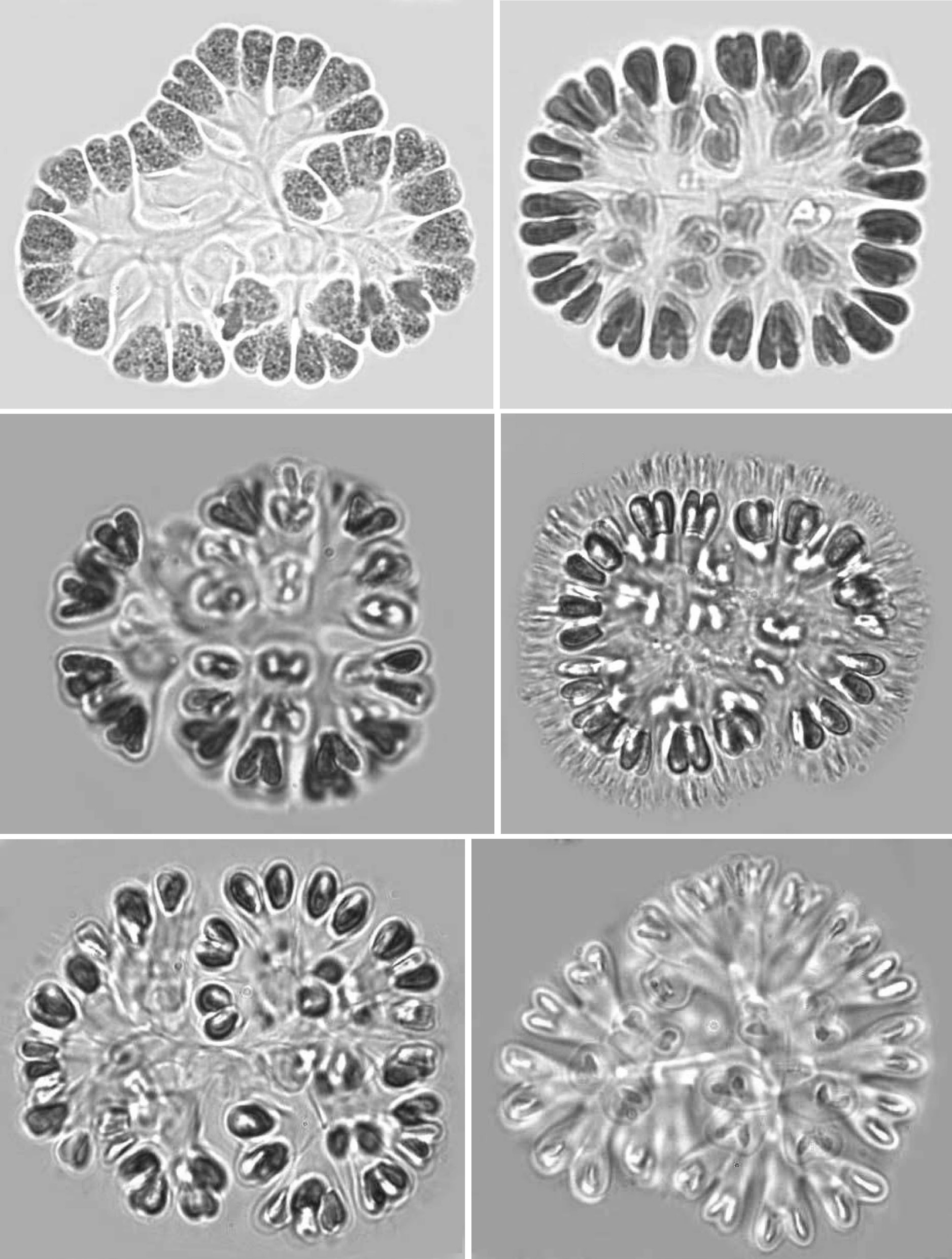
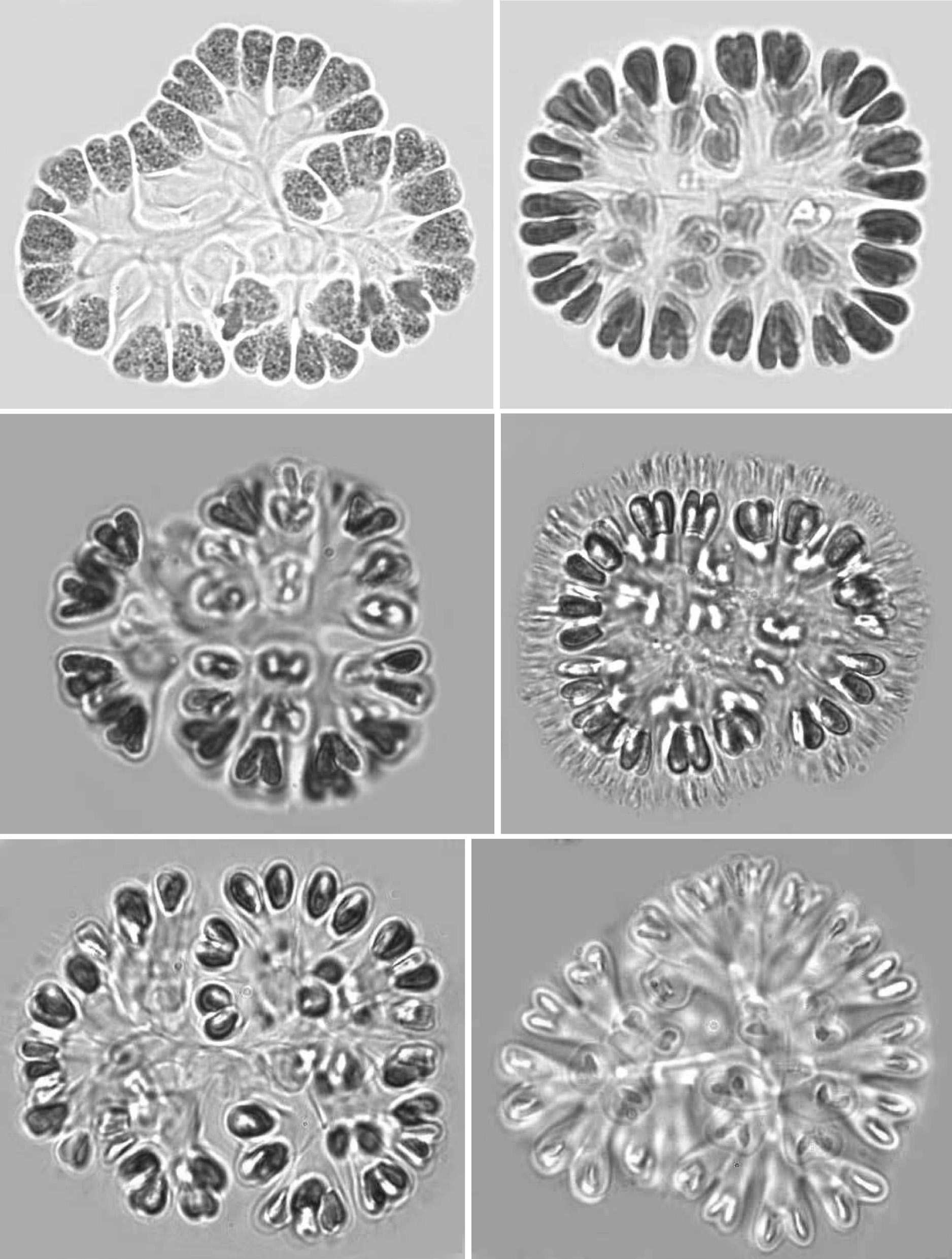
Colonies: Colonies were 25-125 ㎛ in diameter, multicellular(8-256), solitary and often composed of sub-colonies. In shape, they appeared spherical, oblong, ellipsoidal, rectangular or irregular. Colonies appeared blue-green, olive green, pale-green, and, occasionally, pinkish-violet, grey, orange or greenish. Most colonies were found to be 32-celled, but young colonies with 8 cells were frequently observed. Larger colonies had 64 or 128 cells. However, compound colonies, having 128 or 256 cells, with lobed appearance were also present during July and December. The cells in a colony are always multiples of four and may vary from 8, 16, 32, 64, 128 or 256 cells (Pl. 1,figs 1-6).
Cells: Cells were widely obovoid, clubshaped, and, after division, cordiform in shape. They appeared olive-green, grey blue-green, bright blue-green, pale blue-green, yellow green, or pinkish-violet in color. The content varied from homogeneous to granulate at different stages of growth. Cells measured 3-12 ㎛ in width and 5-15 ㎛ in length. Cells were commonly 4-7 ㎛ in width and 5-10 ㎛ in length.
Mucilage and envelopes: During March and October, growing colonies had thin layers of mucilage, but mature and old colonies showed conspicuous and fibrillar gelatinous sheath matrices during July and December. When colonies were pressed under cover slips, central dichotomizing thin tubular stalks were revealed (Pl. 1, fig. 1; Pl. 2, figs 7 & 12; Pl. 3, figs 18 & 21). Also, thread like-structures in different focus appeared (Pl. 1, fig. 2; Pl. 2, fig. 11 Pl. 3, figs 14 & 15). Mature and senescent colonies developed structured envelopes around individual cells as well as a distinct central dichotomizing tubular system (Pl. 2,fig. 12).
Cultures: Efforts were made to isolate a culture in BG-11 medium, but cells did not survive for long. However, colonies grew and reproduced in water of the natural pond habitat for up to three months under laboratory conditions. Then, the cells gradually became discolored and developed more colonial mucilage. In certain colonies,individual cell envelopes with firm dichotomizing stalks were also found in colonies collected from the natural pond habitat. When such colonies were supplemented with BG-11 medium at a 1 : 1 ratio, the colonies became rejuvenated for about two months, and then cells deteriorated and died.
Development of colonies:The young and healthy colonies that were seen mostly in the month of March and October contained eight bi-lobed cells (Pl. 1, fig. 1). The cells were cuneate at the base (towards the center) and broadly rounded towards the outer side. All of these cells appeared bi-lobed due to initial cell division from the outer end (Pl. 1, figs 1 & 2). The cell division process is slow. It appears that, by the time the first series of divisions are completed, the second series of divisions at right angles to the first divisions is already initiated. Then, the colonies possess 16 bi-lobed cells (Pl. 1, fig. 2).Gradually, divisions continue and, by November-December, the colonies have increased in size, and may be composed of sub-colonies and produce up to 256 cells (Pl. 1, figs 1-6). When cell number in a colony increases, the colony may become 2-lobed, 4-lobed or more (Pl. 1, figs 5 & 6), with each lobe containing up to 64 cells.
nies with cells of different shapes (Pl. 3, figs 13 & 14) and dark pigmentations (Pl. 2, figs 8 & 9) were observed during December and January. In many colonies, cells were surrounded by distinct and thick hyaline envelopes and had dichotomizing tubular stalks (Pl. 2, figs 9 & 12). These colonies appeared to be senescent and looked so different that it was possible to identify such populations as new species and relate certain of them (Pl. 2, fig. 12) with the evolution of colonies similar to that found in the genus
Reproduction: When the number of cells in a colony increases, cells grow and secrete a great deal of mucilage. Apparently, pressure is generated, and certain clusters of cells may slip away on slight jerks (Pl. 3, fig. 16). During this study, when a mature colony was pressed, they got separated into four or eight small colonies each containing usually 16 to 32 cells (Pl. 3, figs 18-20). The most interesting feature was that whenever a large colony was pressed, it released several clusters and all were well organized in shape and cell number. This indicated that the pattern of cell division is perfectly synchronized and symmetrical and why clusters give the appearance of autocolonies. Frequently, thick and layered remains of parent mucilage and stalks were clearly seen (Pl. 3, figs 18-21). During June and December, many colonies with enlarged, brownish, granulated cells are covered by mucilage,get settled on the bottom of the tank, and behave as perennating colonies (Pl. 3, figs 15, 17 & 21). During September and March, they come up to the surface and form new colonies.
Taxonomic status:The genus is distinguished from its allied genera of Gomphosphaerioideae by its characteristic paired cordiform cells. The criteria are not well defined for distinctions between species. Species are categorized based on colony size and shape; cell measurement, color and content; thin or thick gelatinous colonial matrix or presence of structured envelope around individual cells; and the presence of a central dichotomizing tubular system. Our two-year observation on enumerable colonies of a single population revealed many variations and structures that have been described for different species (Komarek 1989). In fact, certain completely new shapes of colonies, cells and mucilage envelopes were seen and cannot be compared with any known species (Pl. 2, figs 10-12). One of the researchers (GLT), who has been observing Cyanoprocaryotes for the last over four decades, has never seen such a rich growth of this organism. Previous to this study and on rare occasions, only one or two specimens could be seen on a slide. In this study, records of such wide variations in terms of shape and size, color and mucilage were possible due to the availability of numerous specimens from the natural habitat and continuous observations for more than two years. Further, we searched colonies, including the bottom, lighted, shaded,free-floating, or infested with other filamentous green algae or