This study was conducted to determine the characteristics of gaps and natural regeneration of trees on Mt. Makiling,the Philippines. Canopy gaps in or around two 1-ha permanent plots and on 3-km line transects were investigated. Most of the gaps studied were formed or affected by Typhoon Milenyo, which hit the study site in September 2006. The most frequent mode of gap maker death was snap-off, whereas uprooting was relatively less important. The most frequent gap maker was balobo (Diplodiscus paniculatus) followed by magabuyo (Celtis luzonica) and katmon (Dillenia philippinensis).In contrast, the most frequent gap filler was magabuyo (C. luzonica). At the sapling layer, the most important species was magabuyo (C. luzonica), but there was a high proportion of lianas and palms. Most of the gaps had leaf area index (LAI) values between 3 and 5. A clear trend of a decrease in gap size and an increase in LAI was observed for 2 years from 2007 to 2009. New seedlings emerged very abundantly during the same time period. The rapid changes in the gaps were partially due to the excellent capability of tropical trees to resprout after the crown or stem was damaged by the typhoon. This study on gap dynamics may contribute to a better understanding of the natural regeneration process of trees in tropical rainforests.
The tropical rainforests of Southeast Asia are one of the most diverse terrestrial ecosystems on Earth (Corlett 2009). Forest gaps result in altered physical conditions where they occur (Pickett 1983, Brokaw 1985). Canopy gaps regulate diverse aspects of forest ecology, including species composition (Whitmore 1989, Devoe 1992),maintenance of biodiversity (Connell 1979, Huston 1994), and forest structure itself (Bellingham 1991, Hubbell et al. 1999).
Denslow (1980) suggested that gaps make light and nutrients available, and that these resources are partitioned among colonizing plant species. She argued that the micrometeorological conditions of gaps vary as a function of gap size, and that species may specialize in different portions along this size gradient.
The most important constraint on plant growth and regeneration in tropical forests is low light intensity at the canopy understory (Johns et al. 1996). For temperate and tropical trees, surviving and growing through the juvenile stage are major bottlenecks to achieving canopy tree differences in succession, and species composition and diversity (Clark and Clark 1992). Studies on juvenile tree performance in relation to resource availability in the tropics have provided conflicting results. Generally, shadehouse studies have shown species differences in growth (Popma et al. 1988) and low light survivorship (Augspurger 1984). However, results from field studies are more ambiguous. The eventual occupants of gaps may be either fast-growing pioneer species, which germinate in response to gap formation, or shade-tolerant late-successional species present at the site as seedlings and saplings before the gap was formed. However, the shaded understory is a staging area for advance regeneration that was established prior to gap formation (Uhl et al. 1988) and characteristics of the understory may influence the density and spatial patterns of woody seedlings, which could contribute substantially to patterns of forest composition, structure, and dynamics (MacDougall and Kellman 1992, Montgomery and Chazdon 2001).
Canopy gaps create different environmental conditions affecting the survival and growth of tree seedlings and saplings. The specific objectives of this study were 1) to determine the characteristics of canopy gaps, 2) to determine and compare the environmental conditions in gaps and non-gaps, 3) to measure and compare the rates of seedling diameter and height growth in canopy gaps and in non-gaps, and 4) to determine and compare the emergence and survival rate of seedlings and the presence of saplings as advance regeneration in canopy gaps and non-gaps.
This study was conducted in Mt. Makiling Forest Reserve on south central Luzon Island, the Philippines. The study sites were located at elevations of 400 to 700 m. Natural vegetation in the region has been dominated by many dipterocarp species (Brown 1919).
Most of the original vegetation formed at the mountain base had been selectively logged from 1942 to1944 (Luna et al. 1999). Therefore, the current vegetation belongs to the secondary tropical rainforest, and no specific anthropogenic disturbance has been reported since then. The forest reserve is composed of four vegetation types: the mossy forest zone (900 to 1,109 m a.s.l.), the dipterocarp mid-montane forest zone (100 to 900 m a.s.l.), the grassland zone, and the groforestry zone (Gruezo 1997). Of these vegetation types, the experimental sites were situated in the dipterocarp mid-montane zone. This area has a dry season from January to April and a wet season from May to December. Mean annual rainfall and mean annual temperature are 2,397 mm and 26.5°C, respectively (Luna et al. 1999).
Vegetation in the studied sites was dominated by tree species such as balobo (
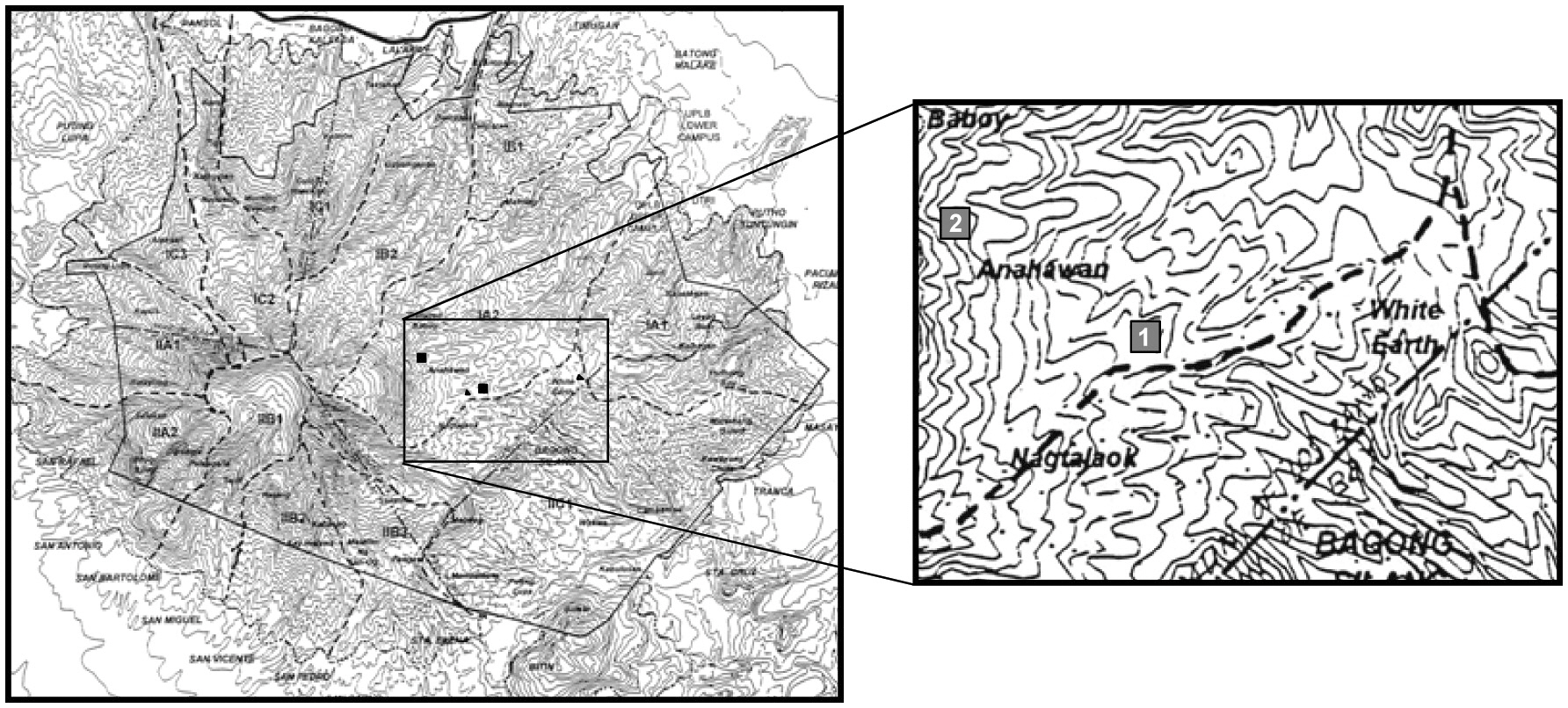
To determine the canopy gap characteristics, gaps within two 1-ha permanent plots (Fig. 1) on Mt. Makiling were selected first, but due to the small numbers, gaps whose centers were located within 20 m from the edge of the permanent plots were also included. Furthermore, six line transects (3 km in total), emanating from the two permanent plots, were set up, and all canopy gaps touching the transects were examined.
The long (L) and short diameters (S) of the canopy gaps were measured for each gap, and gap makers and gap fillers were determined. A canopy gap was defined as an area of perpendicular projections of canopy openings caused by the death of one or more canopy trees (Runkle 1982). Clinometers were used to determine gap boundaries. Gap shape can be circular or irregular, but these were mostly oval (elliptical). All gaps were regarded as oval. Gap size (A) was calculated as follows (Cho 1992):
A = πLS/4
Gap size was measured in April 2007 and again in April 2008, and the changes in gap size over the year were calculated.
Information about the gap makers and their replacers (gap fillers) is important when tracing the successional trend. The species and number of all gap makers and their replacers were recorded for each gap (Cho 1992).
The causes of gap formation and modes of death of gap makers are diverse. First, the causes of gap formation were classified into a primary cause by typhoon or other factors and a secondary cause by fallen trees. Second, modes of death of gap maker trees were classified into uprooted, snap-off, standing dead, branch breaks, and leaning.
>
Seedlings and saplings in gaps
Survival, recruitment, and growth of tree seedlings was determined for 1 year by identifying the tree seedlings and measuring the growth of basal diameter of stem at 1 cm above the ground, and the growth in height for 1 year from April 2007 to April 2008. To determine the relative importance of advance regeneration, saplings were regarded as trees when 1 cm ≤ diameter at breast height (DBH) < 5 cm. Seedlings were regarded as small trees when DBH < 1 cm.
Two sets of canopy analyzers (LAI 2000; Li-Cor, Lincoln, NE, USA) were used to determine LAI as a measure of canopy openness of a gap. One was placed at a reference site in an open area and another at the sites where LAI data were needed (in the gap or non-gap) within the permanent plots or on transects. At each site for LAI measurement, readings of light intensity from eight different azimuth directions on the canopy analyzer were compared with those on the canopy analyzer at the open reference site, and then the eight LAI values were averaged for the final LAI calculation for the site.
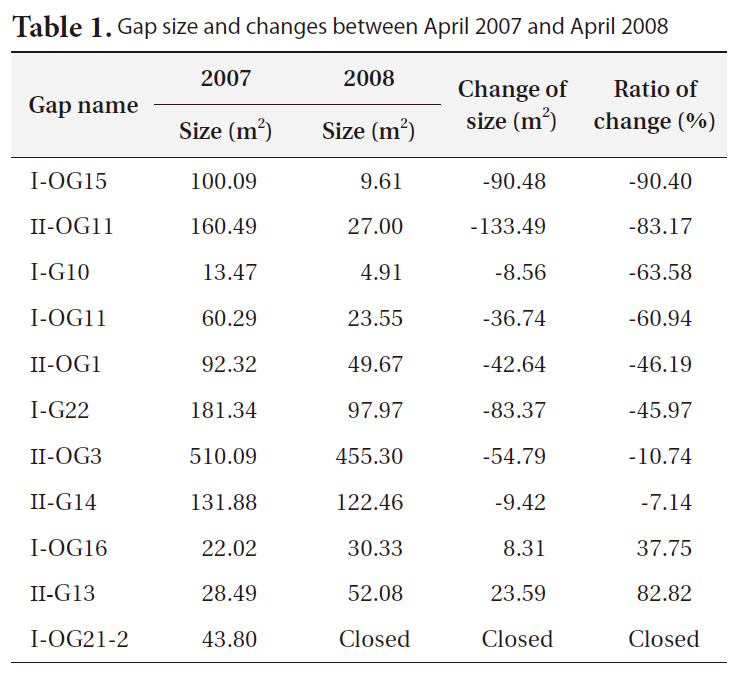
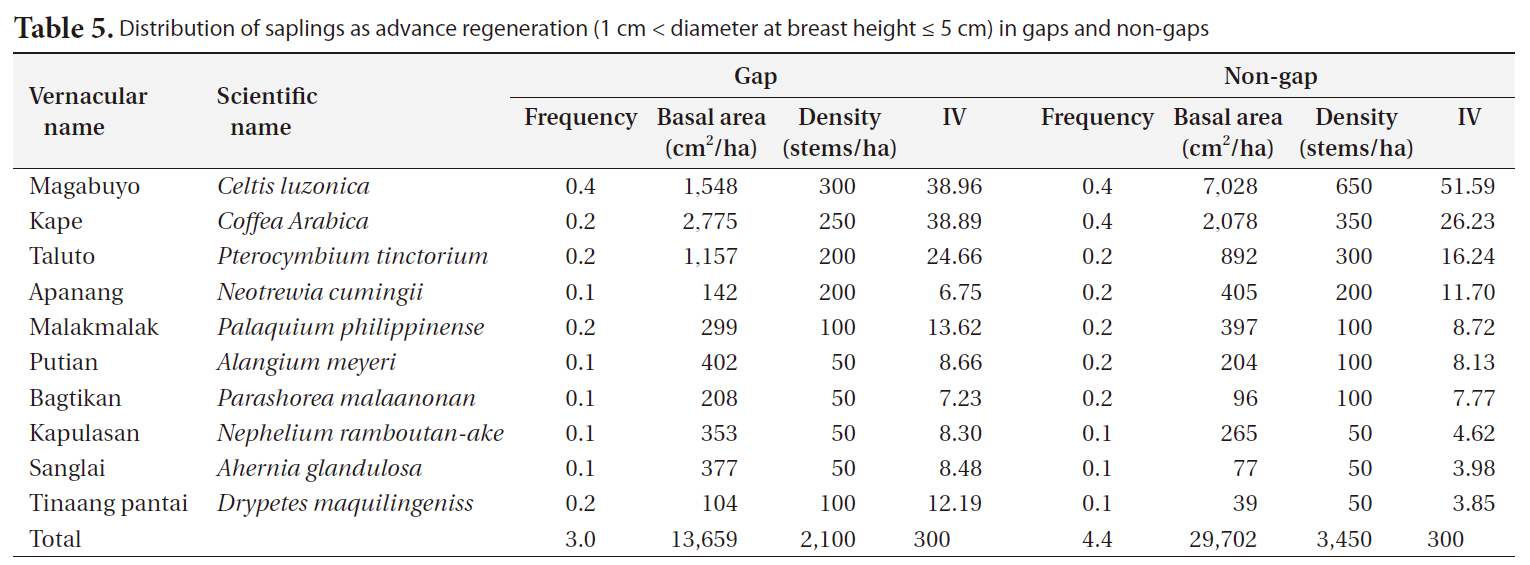
Gap size generally decreased between 2007 and 2008 (Table 1). However, large variations in the change in gap size from a large increase in size (+83%), to little changes (-7 to -10%), to a big decrease (-90%), or to complete gap closures were observed.
Bigger gaps had a smaller decrease ratio than smaller gaps due to the relatively small growth compared to the diameter of gaps. Some gaps showed an increase in size, but the cause could be a measurement error, as gaps are very complex in shape and measuring sometimes requires subjective judgments.
[Table 1.] Gap size and changes between April 2007 and April 2008
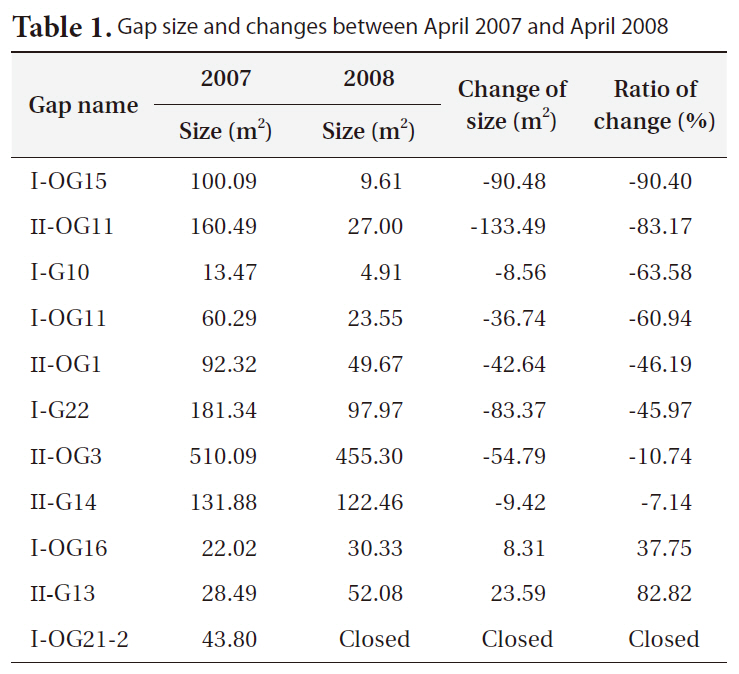
Gap size and changes between April 2007 and April 2008
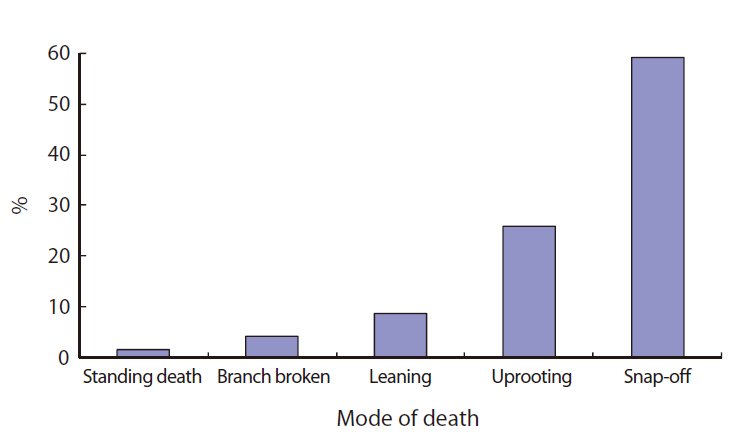
Most of the gaps studied were formed or affected by Typhoon Milenyo, which hit the study site in September 2006. A canopy tree can fall to the ground directly by a disturbance (primary cause), but some trees can die as a result of another fallen tree (secondary cause). More than 60% of the gap makers died directly due to the typhoon, but a relatively higher proportion of gap makers fell to the ground because of other large trees that fell (data not shown). Canopy trees with heart-rot in the main trunk can be easily broken by a disturbance. Only 8% of the gap makers on Mt. Makiling had heart-rot before they died (data not shown).
About 60% of the gap makers died due to breakage in the main trunk caused by Typhoon Milenyo in 2006, and another 30% were uprooted (Fig. 2). The trees that are leaning are different from uprooted trees in that they are still alive and have high potential to produce sprouts from their main trunks. Many of the gap makers with broken stems and branches were able to survive and were recovered from the damage by quick resprouting.
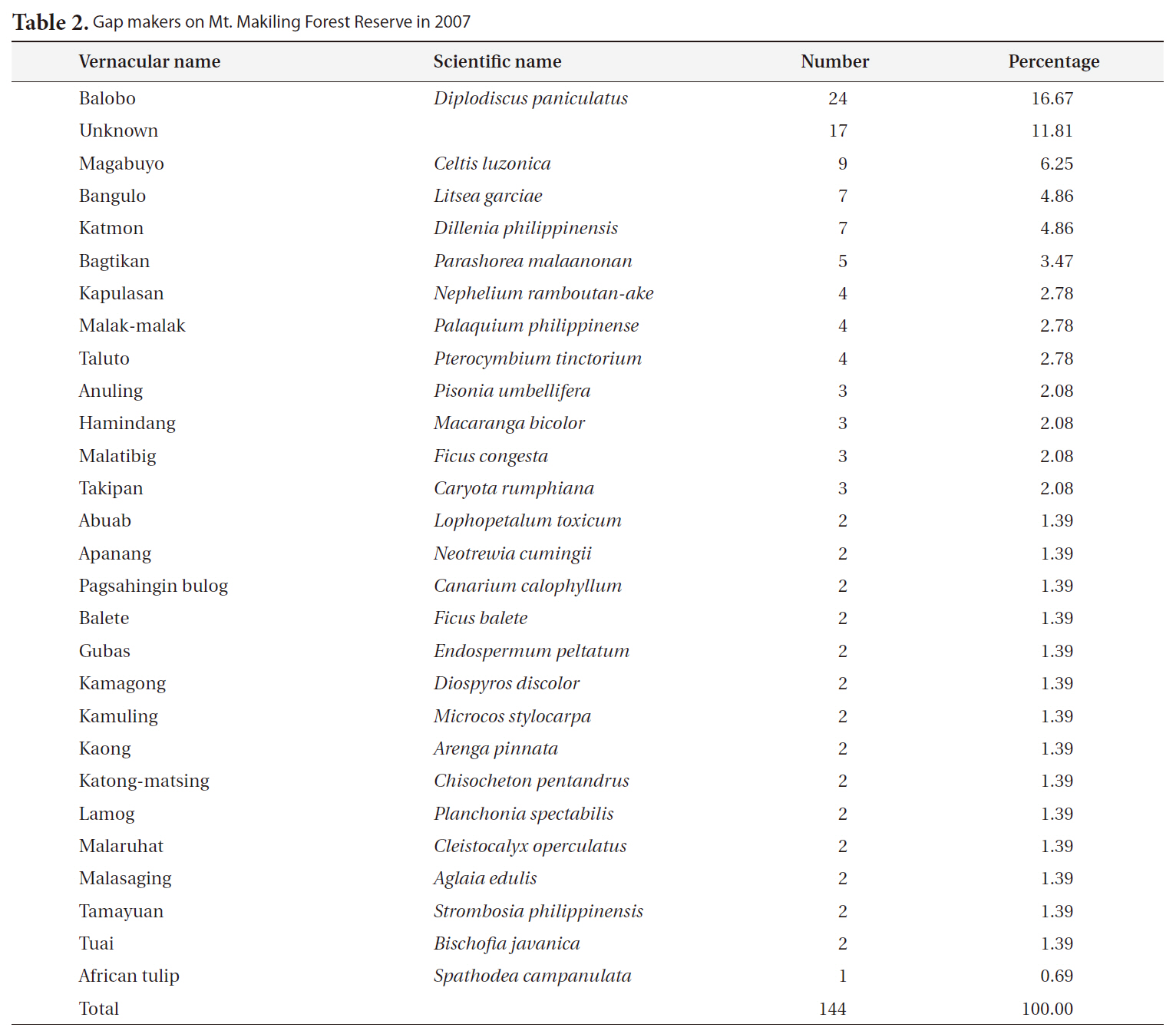
The most frequent gap maker on Mt. Makiling was balobo (
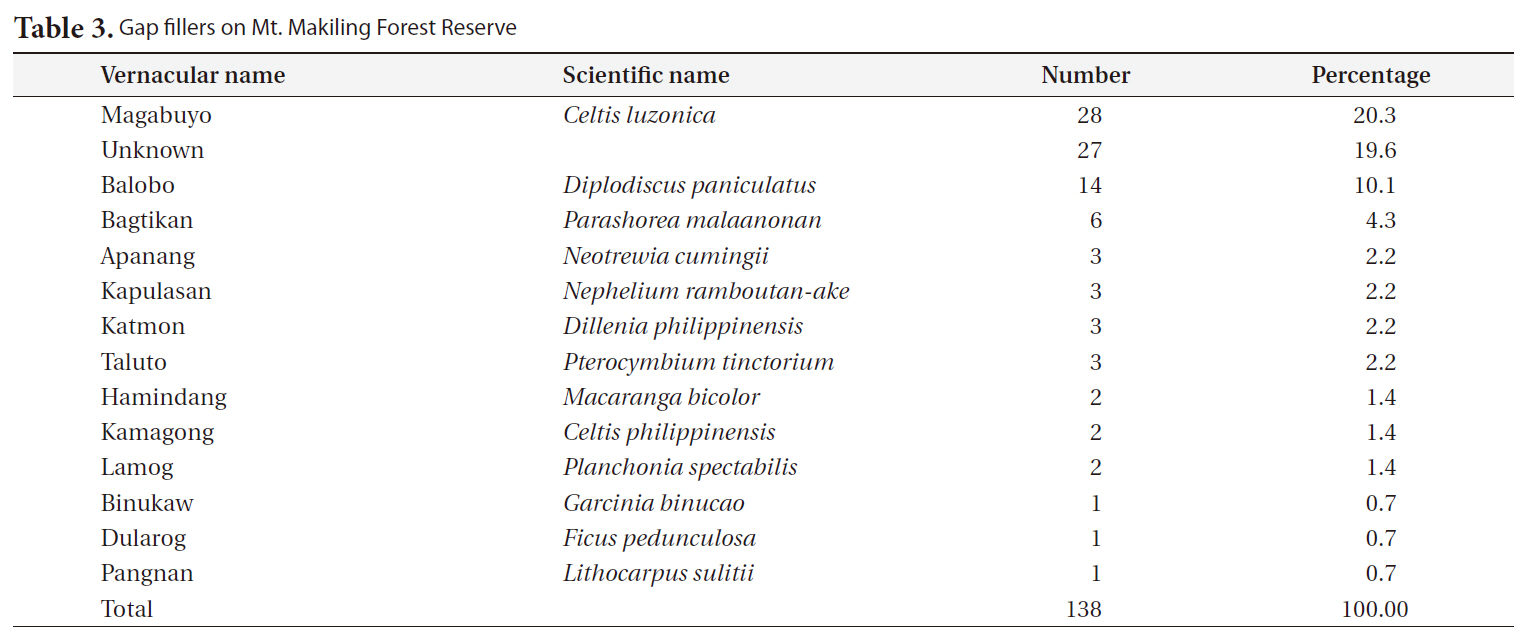
The most frequent replacers for gap makers were magabuyo (
>
Seedlings and saplings in gaps
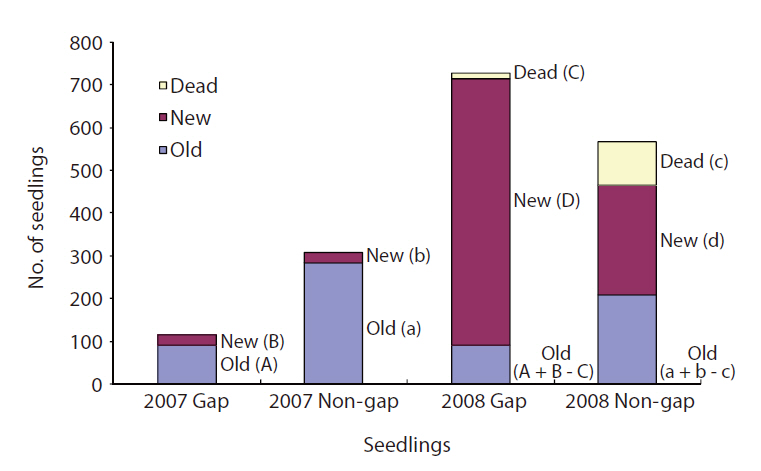
Seedling survival and mortality were determined in 2007 and 2008 in gaps and non-gaps, and new seedlings and existing seedlings were counted each time. Non-gaps had more seedlings than gaps in 2007. However, seedling number in the gaps was three times higher than that in non-gaps in 2008 (Fig. 3). The mortality rate of seedlings was seven times higher in non-gaps than in gaps. In 2008, most of the seedlings in gaps were new (Fig. 3).
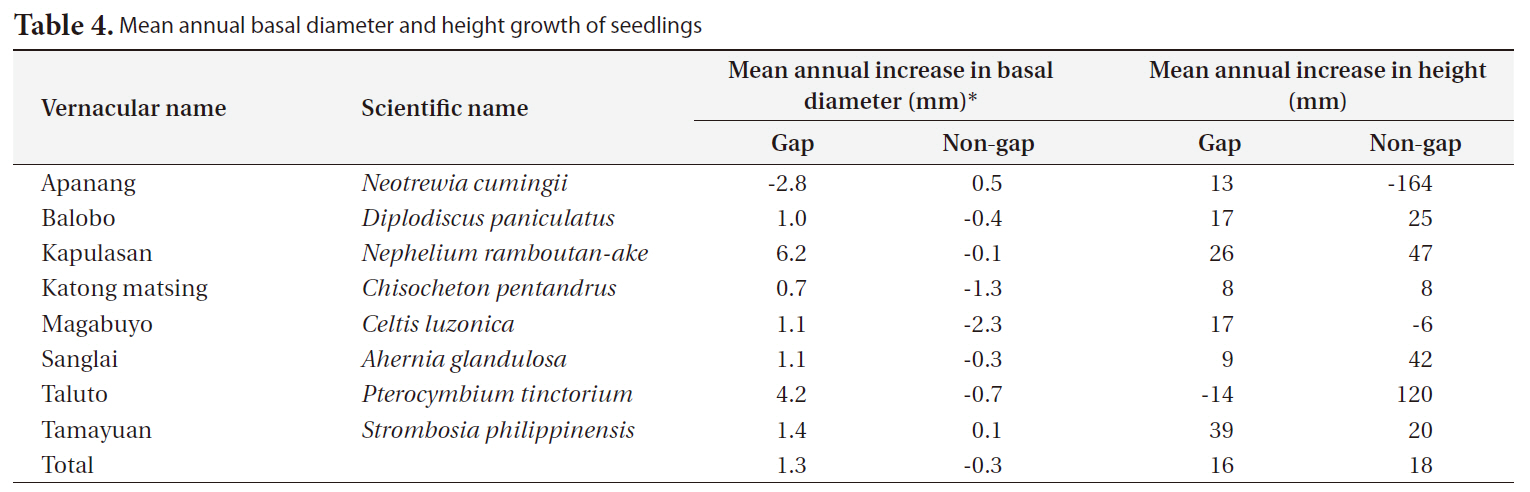
The annual diameter growth of seedlings was generally higher in gaps than in non-gaps. In gaps, particularly the mean annual diameter growth of kapulasan (
Gap and non-gap sites showed similar sapling distributions. The highest frequency of saplings in gaps and non-gaps were in the order of magabuyo (
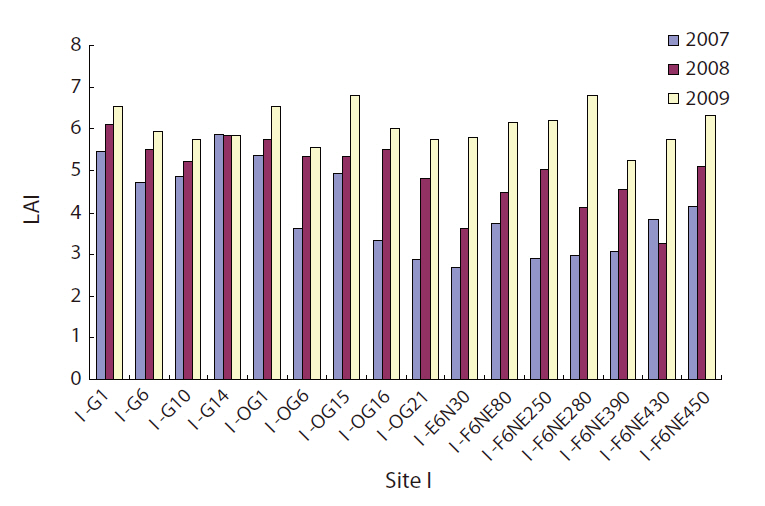
The LAI will be zero when an area is completely open without any vegetation, and a forest with a closed canopy will have a very high LAI. A large increase in LAI was observed for the 2 years from 2007 to 2009 both in gaps and in non-gaps (Fig. 4), and the increase was highly statistically significant (
[Table 2.] Gap makers on Mt. Makiling Forest Reserve in 2007
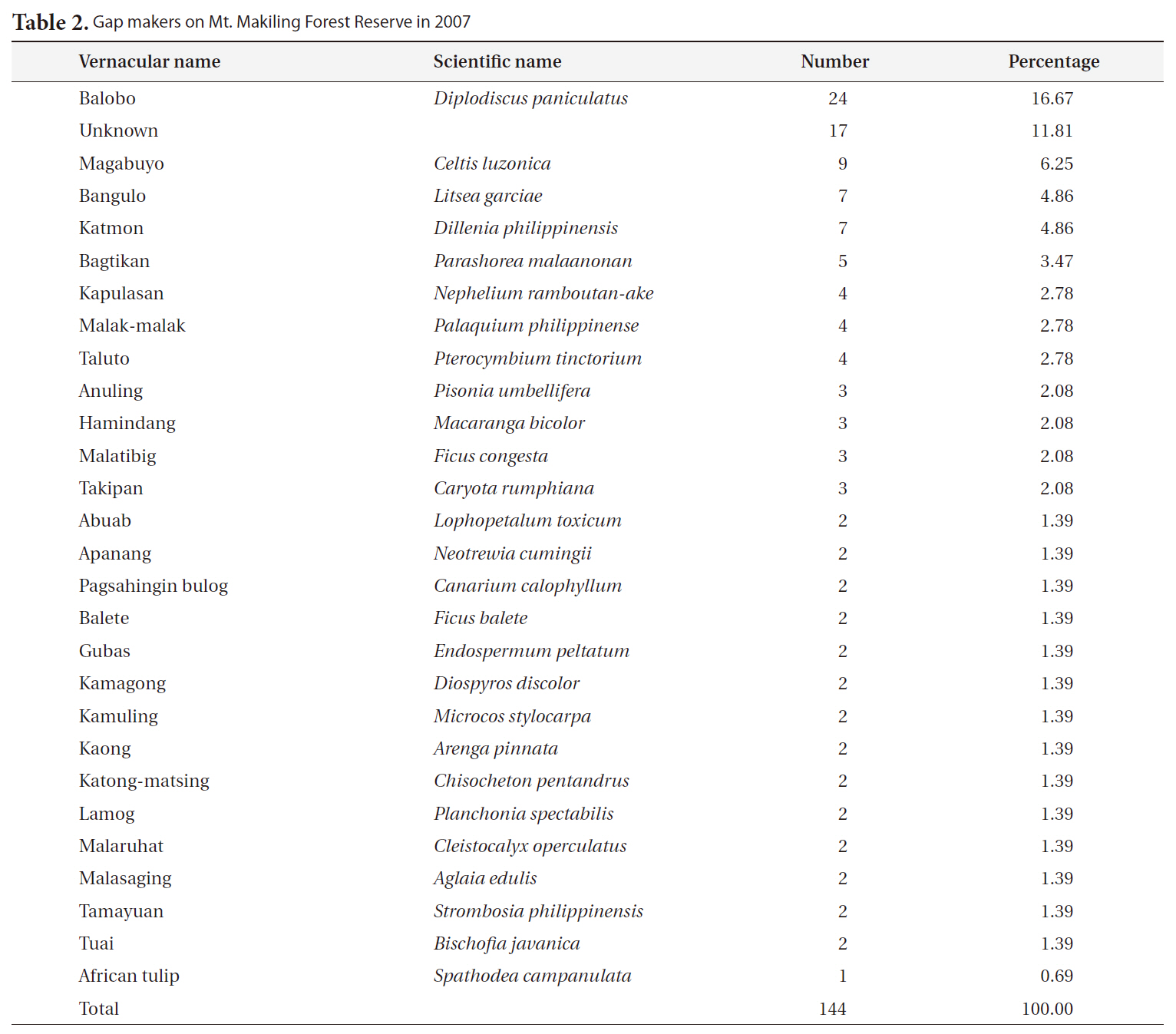
Gap makers on Mt. Makiling Forest Reserve in 2007
Gaps undergo continuous disturbances for extended periods. In forests, some species require large gaps to regenerate and show indications of decreased growth from gap center to edge to understory with little relationship to aspects (Runkle et al. 1995). Gap dynamics are very important for understanding forest structure and natural tree regeneration.
The mode of gap formation is determined by the kind of forest and the superior disturbance regime (Foster 1988). Typhoon Milenyo severely hit this area on September 26, 2006 with very strong winds and heavy rains in a relatively short period of time. Thus, the status of
[Table 3.] Gap fillers on Mt. Makiling Forest Reserve
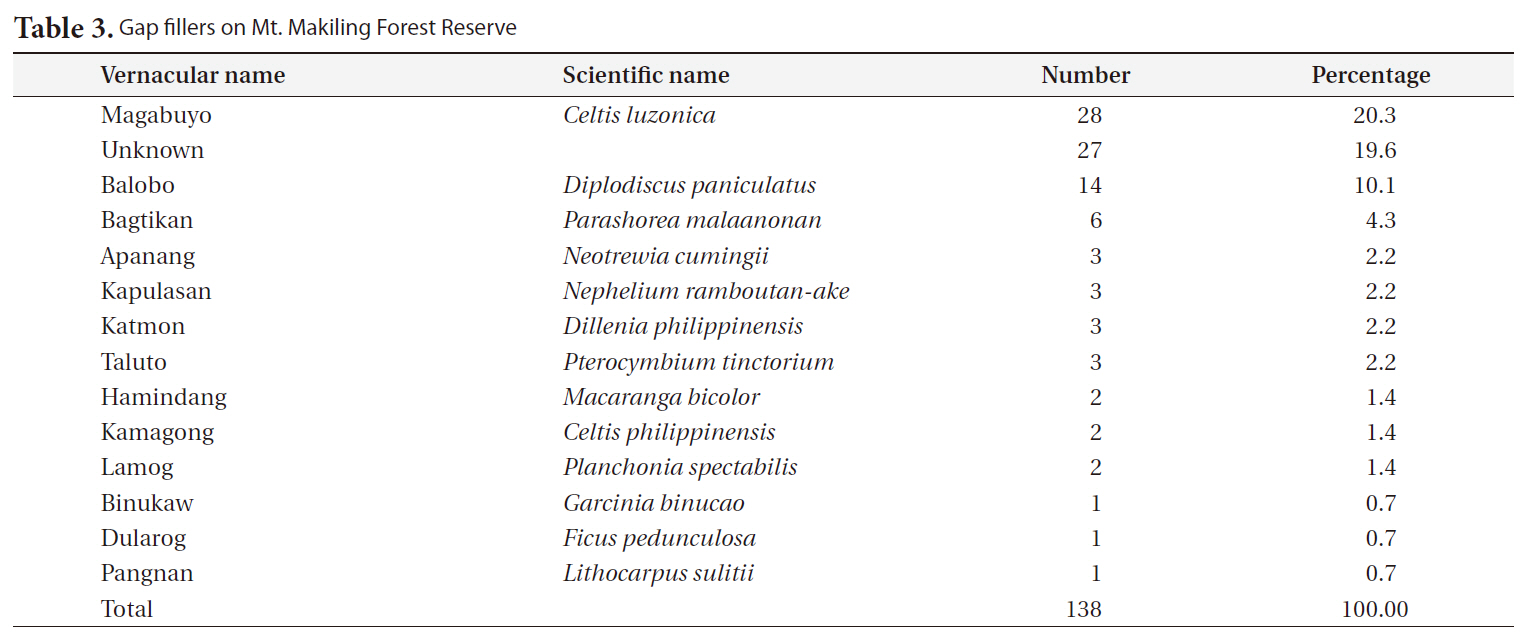
Gap fillers on Mt. Makiling Forest Reserve
[Table 4.] Mean annual basal diameter and height growth of seedlings
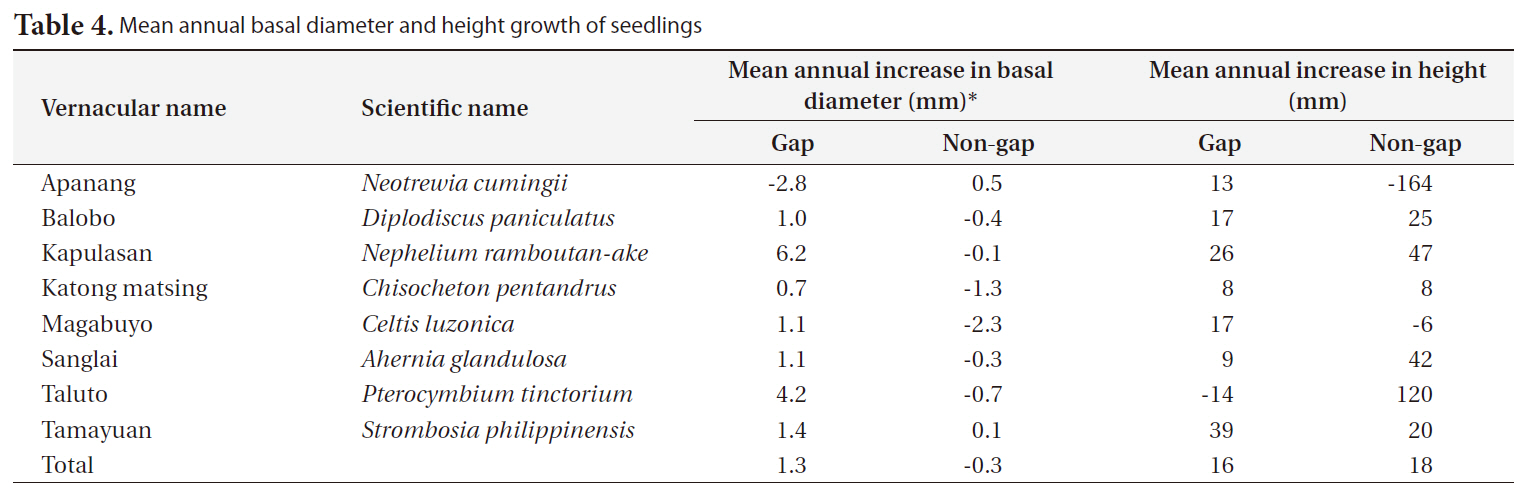
Mean annual basal diameter and height growth of seedlings
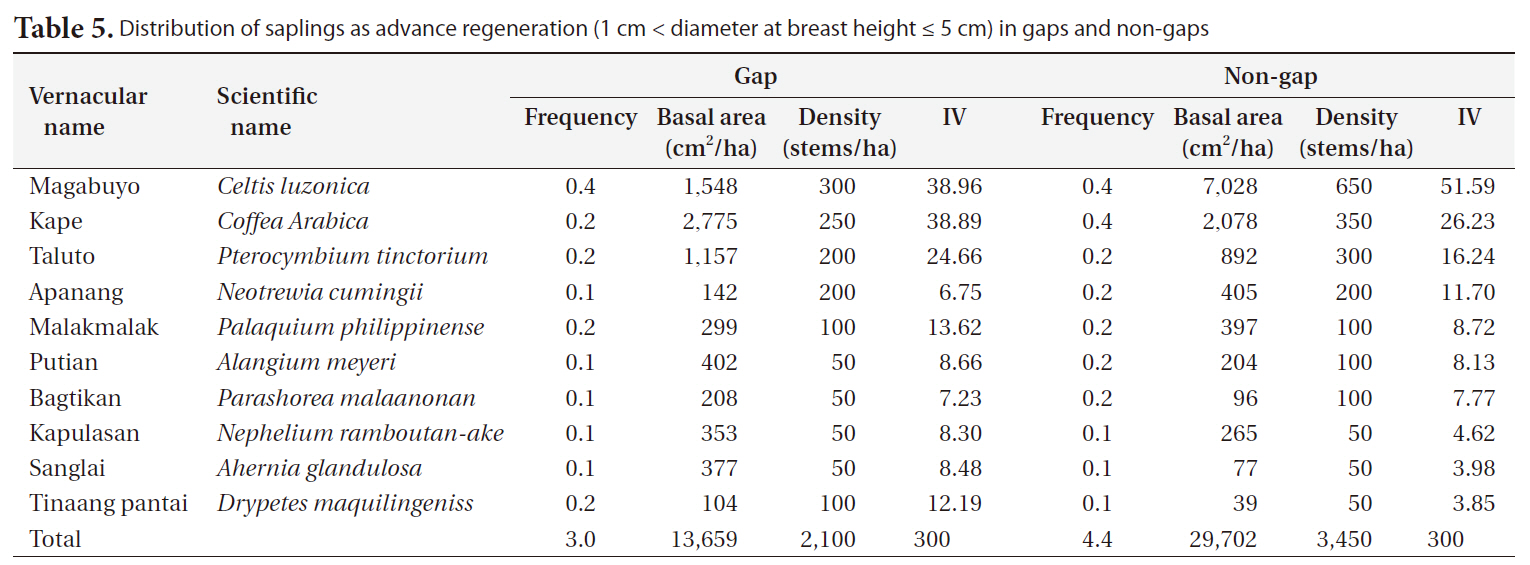
Distribution of saplings as advance regeneration (1 cm < diameter at breast height ≤ 5 cm) in gaps and non-gaps
gap makers was affected by Typhoon Milenyo on Mt. Makiling. The effect of typhoons or hurricanes may differ between temperate and tropical forests. First, temperate and tropical storms vary in frequency, magnitude, and intensity. Secondly, broad-scale typhoon characteristics, particularly peak wind direction, interact with local topography to influence the pattern and type of damage observed at the stand level (Boose et al. 1994).
The major cause of death on Mt. Makiling was the typhoon of 2006. However, the rainforest canopy trees were distributed very closely to their neighbors, had a large DBH, and high stature and were connected by many lianas. Thus, it might be easy for the neighboring canopy trees to get hit by other trees falling from typhoons. A relatively higher percentage of gap makers were downed by neighboring trees falling on them on Mt. Makiling in 2006.
Few gap makers had heart-rot before they died in this study. This was probably due to the rapid growth and to the ability of canopy trees to resprout in tropical forests. In contrast, many old and large trees develop heart-rot in temperate forests, and this can be a predisposing factor for easy treefalls (Cho 1992).
Direct damage due to many disturbances has an effect on the snap-off of large branches rather than on the whole tree (De Steven et al. 1991). In the tropical wet forest at La Selva, Costa Rica, 90% of the gaps were formed by uprooting (Hartshorn 1980), while in the tropical moist forests of Mpassa, Gabon and Barro Colorado Island, Panama, snapping prevailed (Lawton and Putz 1988). On Mt. Makiling, the most frequent mode of death for gap makers was snap-off. The presence of buttress roots is a factor commonly proposed to highly influence death by snapping due to reduced uprooting (Gale and Barfod 1999).
Balobo (
Gaps generally decreased in size or completely closed in the year from April 2007 to April 2008 on Mt. Makiling. During the first 2 years after Typhoon Milenyo, a very big change in the forest was evident. The rapid decrease in gap size and the increase in LAI indicated that a natural recovery from damage by the typhoon was occurring rapidly on Mt. Makiling and much faster than that in temperate forests. If gap openings are long and narrow, canopy closure can occur by lateral expansion of the gap-bordering canopy or subcanopy trees (Uhl et al. 1988). In a very short period of time of 1 year, the reduction in gap size must have been due to the lateral growth of trees in the gaps on Mt. Makiling.
Natural, undisturbed forests are self-maintaining through dynamic processes of regeneration including recruitment, growth, and mortality. Trees die and get replaced with new recruits; thus, setting the vegetation into a dynamic equilibrium (Swaine et al. 1987). Most tropical forest tree species do not exhibit species to species replacement. Furthermore, the present state of seedling and sapling species composition will decide the future canopy tree species (Runkle 1981). Forest growth models frequently assume that occupancy of space prior to gap formation is a good predictor of future canopy composition (West et al. 1981). Forest gaps generally serve to boost species already present in the understory but do not create opportunities for a new set of species (Runkle 1998). On Mt. Makiling, the most frequent species in the sapling layer in gaps was magabuyo (
A positive relationship between the high-light growth rate and low-light seedling mortality occurs in both temperate and tropical forests. This was true in this study, but the most significant difference between gaps and non-gaps after a big typhoon was seedling emergence and survival rates. Growth responses appear to be light mediated. Large multiple-treefall gaps receive considerably more light at ground level than smaller single-treefall gaps, and those smaller gaps receive more light than a closed-canopy forest (Uhl et al. 1988). Ground vegetation cover in the gaps can also affect tree regeneration indirectly by providing habitat and food for herbivores that feed on seeds and young trees (Babaasa et al. 2004). Light availability in gaps is in reverse proportion to LAI.
This study on gap dynamics will contribute to a better understanding of the natural regeneration process of trees in tropical rainforests, but a more detailed study on the physiology of trees is necessary, particularly one on the effects of light on individual tree species.