Fertilization practices alter the aboveground ecosystem and the community of soil organisms via increased disturbances and changes in habitat. In conventional agroecosystems with considerable disturbance or high fertilizer input, the soil organisms with short life cycles reproduce rapidly, and frequently increase in population quite rapidly (Berkelmans et al. 2003), and repeated fertilization alters the composition of plant species and ameliorates weed diversity (Hobbs and Huenneke 1992,Yin et al. 2006). In low-input systems with reduced or organic fertilization, the populations of long-lived and slowly reproducing organisms tend to increase, and more diverse weed communities are detected (Berkelmans et al. 2003).
The importance of free-living soil organisms in relation to organic matter decomposition, nutrient cycling,and nutrient uptake by plants is being increasingly recognized.The population density, activity, and diversity of free-living soil organisms are generally correlated with the decomposition rate of organic matter (Bettiol et al.2002). Bacteria and fungi perform important roles in the chemical breakdown of organic materials, and these microorganisms also constitute a source of nutrients in the process of nutrient cycling (Coleman et al. 2004). Nematodes and microarthropods comprise a vast majority of the micro- and mesofauna in soil (Coleman et al. 2004).Free-living nematodes are beneficial for the decomposition of organic matter and for nutrient recycling in soil (Mikola and Sulkava 2001). Microarthropods, including collembola and mites, fragmentize plant residues (Filser 2002).
Plant community and diversity are driving forces that alter belowground ecosystems (Hooper and Vitousek 1997, De Deyn et al. 2004). Particularly, the weed community affects microflora, microfauna and mesofauna in the agroecosystem (Garrett et al. 2001, Queneherve et al. 2006), and frequently evidence symbiotic or antagonistic relationships with organisms in soil (Morgan et al. 2005). Considering the interaction between above- and belowground ecosystems, the response of the communities of weeds and soil organisms should be simultaneously investigated in order to evaluate the impacts of fertilization cessation on the system.
The principal objective of this study was to assess the effects of the cessation of fertilization on the communities of soil organisms and weeds. This study should provide useful insights into the interactions occurring between aboveground weed communities and belowground fauna in different soils harboring different levels and types of nutrients. The effect of the cessation of organic fertilization (CO) was also assessed in an effort to compare the results of this approach to that of the cessation of applying inorganic fertilizer. In order to obtain information regarding the impact of the external input of fertilizer, complete cessation of the input would be an effective alternative method.
The experiments described herein were conducted between July 2004 and June 2006 in the Experimental Farm of the University of Tokyo (35°43′ N, 139°32′ E). The soil type was a Humic Andosol comprised of 29% sand, 38% silt, and 33% clay. The annual mean temperature is 15.9°C and the annual precipitation is 1,466 mm/y. Six treatments (3 fertilized and 3 ceased treatments) were tested. The following 3 treatments have been maintained since 1993, each treatment applied to a plot size of 8 m × 50 m: conventional fertilization (CF), reduced fertilization (RF), and organic fertilization (OF). These plots were managed under a 1-year double-cropping system with corn (
>
Experiment in the corn field
Inorganic NPK fertilizer was applied at a rate of 42 kg N/ha, 23 kg P/ha and 46 kg K/ha to the CF and RF plots before seeding. Additional fertilization was conducted via the application of the same amount of inorganic fertilizer as a top dressing to the CF plots on August 13, 2004 and August 17, 2005. The OF plots were treated in late June every year with composted manure (cow manure and plant straw), which contained (on average) 69% water and 2.3% nitrogen on a dry weight basis, at a rate of 80 t ha?1 y?1. Corn (Z.
>
Experiment in the wheat field
Inorganic NPK fertilizer was applied immediately prior to the seeding of winter wheat at a rate of 84 kg N/ha, 55 kg P/ha and 93 kg K/ha in the CF plots, and 50% of this amount was applied in the RF plots. On November 25, 2004 and November 18, 2005, winter wheat (
The aboveground weed biomass was measured between October 2 and October 8 in the corn field and between June 1 and June 7 in the wheat field in both years. The biomass was estimated in 3 quadrants per plot, each having an area of 71 cm × 50 cm in corn and 54 cm × 50 cm in wheat. The weeds collected in each plot were cut at ground level and separated according to species. They were dried at 80°C, and their dry weights were measured.
In order to carry out evaluations of the soil organism populations, soil samples were collected on October 2, 2004 and October 1, 2005 from the corn field and on June 2, 2005 and June 3, 2006 from the wheat field. Interrow soil samples were collected from four places with a depth of 0-10 cm using a 4-cm-diameter boring sampler, and combined into a single composite sample for each plot.
The activity level of microorganisms was evaluated via the substrate-induced respiration (SIR) technique. Fresh soil equivalent to 10 g (dry-weight) was mixed with 20 mg of glucose and incubated at 22°C. The CO2 evolved at between 2 and 4 h of incubation was measured with an infrared CO2 analyzer (Model LX-720; Iijima, Tokyo, Japan). Nematodes were extracted from 10 g of fresh soil over 48 h using a Baermann funnel, then counted under a dissection microscope. Microarthropods, including mites and collembolan, were extracted over 48 h using a Tullgren funnel with a 2-mm mesh under fluorescent lamps.
The effects of fertilization treatments were analysed via ANOVA followed by Tukey’s test using SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA). The results of microbial SIR and the populations of nematodes and microarthropods were compared, and correlation coefficients between the aboveground weed biomass and the soil organisms were calculated.
>
Changes in weed community occurred due to the cessation of fertilization under corn cultivation
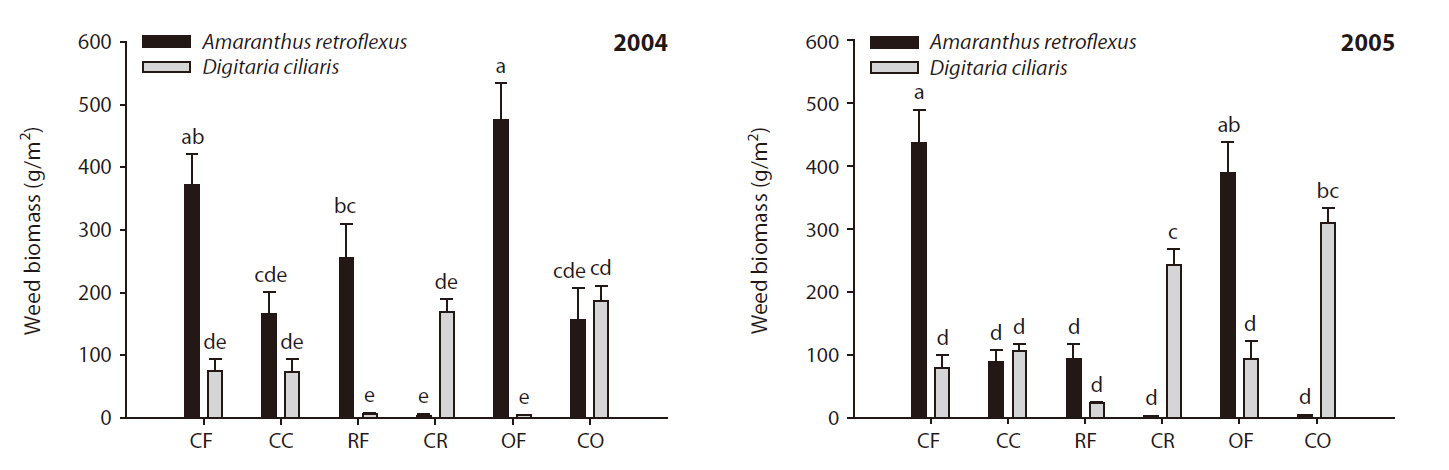
The effects of different fertilization treatments on weed community dynamics in corn are shown in Fig. 1.
aboveground weed biomass was greater in the fertilized plots than in the fertilization-cessation plots, except in the OF plots. Obvious changes in the weed community were observed between
>
Changes in weed community occurring due to the cessation of fertilization under wheat cultivation
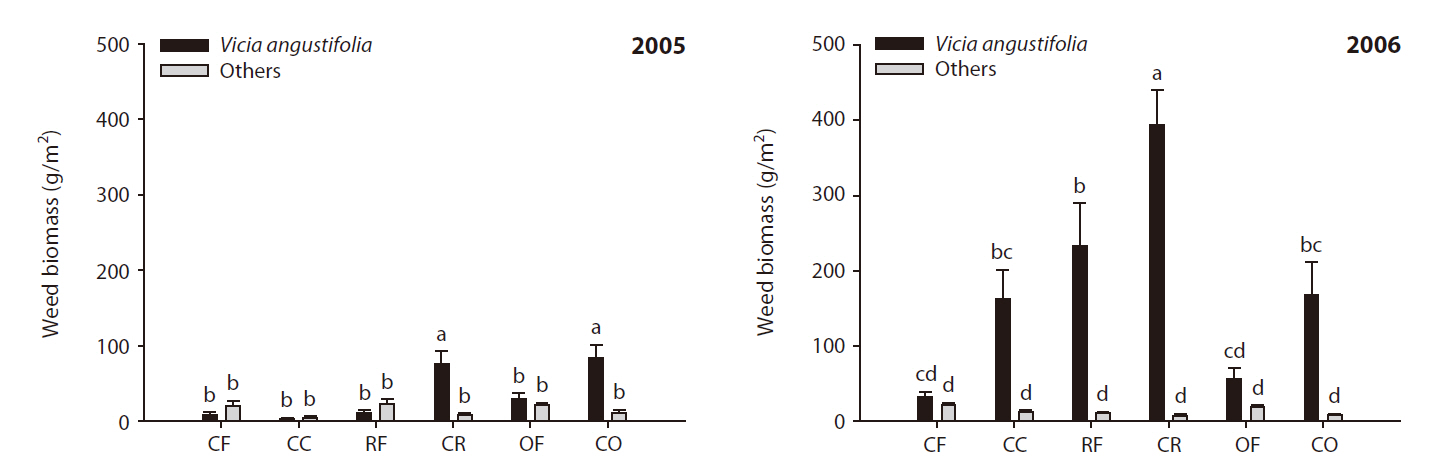
Weed communities in wheat were affected by the cessation of fertilization (Fig. 2).
>
Changes in SIR and population density of nematodes and microarthropods
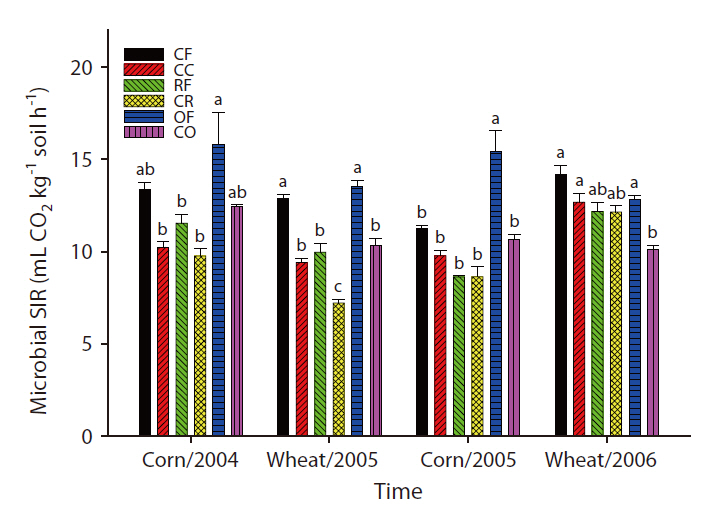
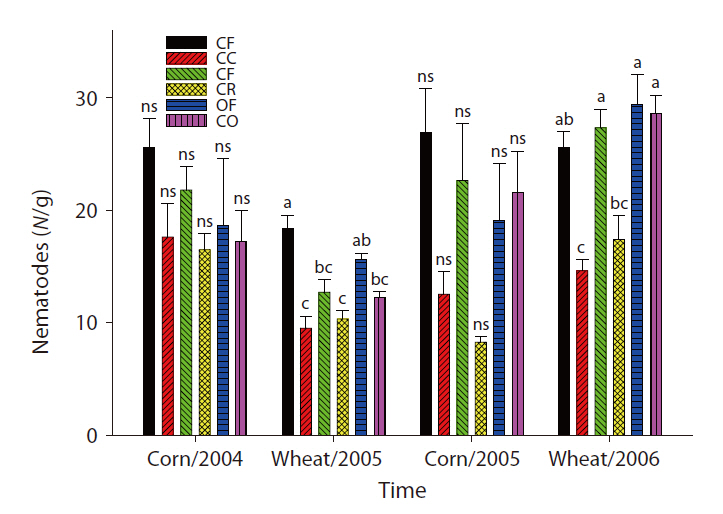
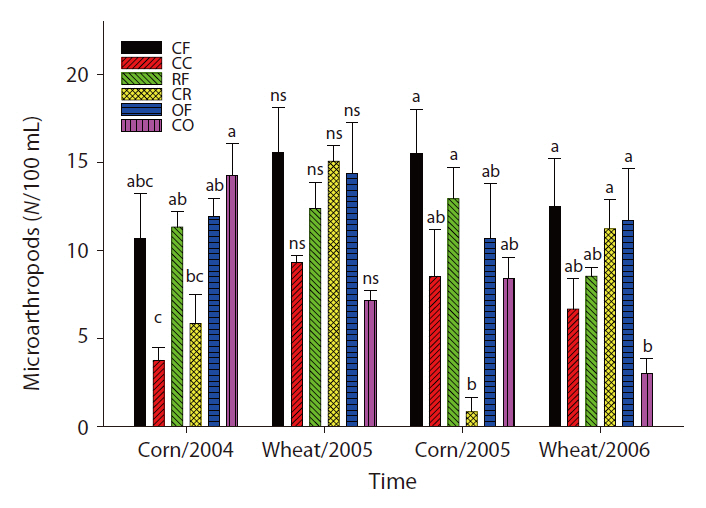
The overall microbial SIR decreased after the cessation of fertilization (Fig. 3). In the CC and CR plots, the population density of nematodes decreased after the ap-
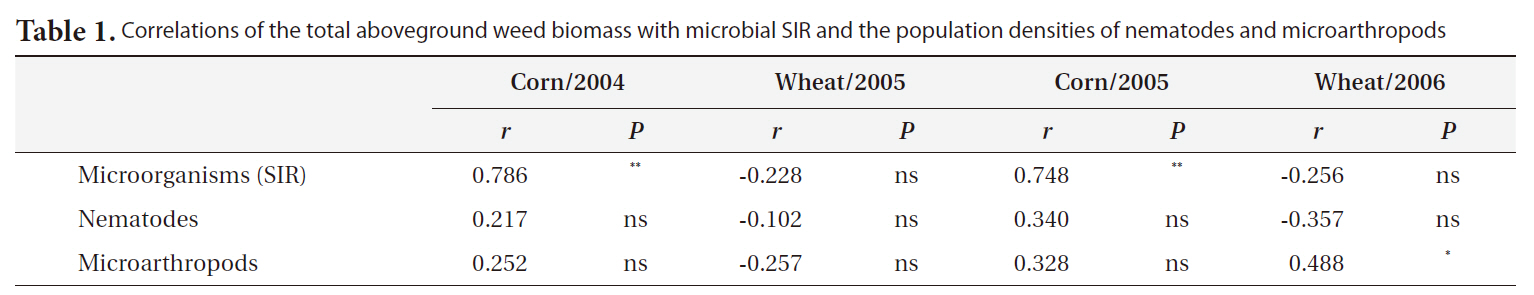
plication of inorganic fertilizer ceased (Fig. 4). One result worth mentioning was that in the CO plots, the nematode population changed slightly after the cessation of organic fertilization. The population density of microarthropods was decreased somewhat after the application of organic or inorganic fertilizer ceased (Fig. 5). With regard to corn cultivation, the total aboveground weed biomass was correlated strongly with microbial SIR in both years. The aboveground weed biomass was positively correlated with the population density of microarthropods only in wheat cultivation in 2006 (Table 1).
The infestation of
In the wheat field, the most conspicuous change noted was the rapid increase in the population of
The colonization of a specific plant may alter the belowground ecosystem (Keith et al. 2006, Pritekel et al. 2006). The composition of plant species is an important factor that impacts the development of nematode communities, and large populations of herbivorous and bacterivorous nematodes were detected under grasses and legumes, respectively (Viketoft et al. 2005). Different species of leguminous plants exerted different effects on the
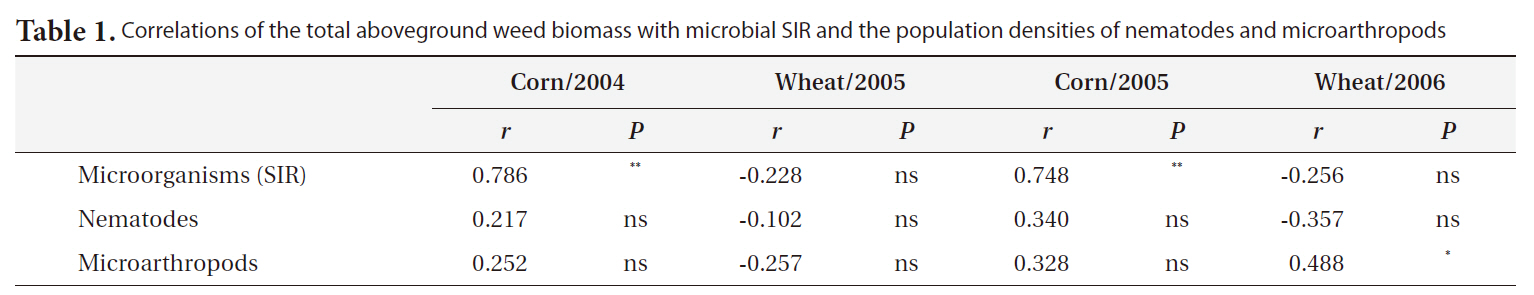
Correlations of the total aboveground weed biomass with microbial SIR and the population densities of nematodes and microarthropods
abundance and constituencies of nematodes (Villenave et al. 2003, Hoschitz and Kaufmann 2004). However, it remained unclear whether soil organisms were influenced by plant specificity, and particularly by
No consistent trend in the effects of the cessation of fertilization on the responses of soil organisms were detected in this study. A reduction in the amount of fertilizer applied exerted inconsistent effects on soil organisms in previous studies. Reduced inorganic fertilizer input in a riparian field favoured the growth of microorganisms (Ettema et al. 1999), but eliminating the application of organic fertilizer negatively affected microorganism growth (Ritz et al. 1997). The cessation of fertilization exerted no effects on the soil microbial community of a meadow, and in a long-term experiment, the fungi/bacteria ratio was higher in the fertilization-cessation grasslands than in the fertilized grasslands (Bardgett and McAlister 1999). The reduced nutrient levels in the soil also affected the nematode communities (Smolik and Dodd 1983, Dmowska and Ilieva 1995), and the population density of total nematodes increased following the cessation of fertilization (Hanel 2003).
The strong correlation between the total aboveground weed biomass and microbial SIR in corn implied that the cessation of fertilization might affect microorganisms indirectly through changes in the weed community. Although no significant correlations were determined to exist between the population density of nematodes and the total aboveground weed biomass, the increase in living plant biomass can amplify the abundance of nematodes, and may be associated with the input of carbon to soil. Aboveground plant growth was correlated with nematode abundance and microbial biomass (Yeates et al. 2004). The reduction in aboveground productivity with an increase in time after the cessation of fertilization was found to coincide with a reduction in nematode population density (Olff and Bakker 1991). Herbivorous nematodes can be affected by food source quality when nutrient concentrations decreased and defensive compounds increased under nutrient deficiency conditions (Verschoor et al. 2001). In wheat cultivation in 2006, it is worth noting that the population density of microarthopods was highest in the CR plots, despite the cessation of fertilization. The increased population appeared to have been affected by the feedback effect of the increased biomass of
In conclusion, the responses of weed communities to the cessation of fertilization between inorganic and OF systems were found to differ only slightly. The distribution of the weed community was governed by species that had adapted to low nutrient levels. It is difficult to come to any definitive conclusion regarding the effects of fertilization cessation on soil organisms, because so many different responses were observed. However, the total aboveground weed biomass in corn was fairly clearly correlated with microbial respiration and also partly correlated with the microarthropod population density. The mutual relationship between