The removal of toxic heavy metal ions from wastewater has been widely studied in recent years. Chromium is one of the heavy metals of immediate concern in many countries. Industrial effluents from tanning, electroplating, painting and textile industries contain chromium species above the maximum allowable contaminant level [1]. Although both trivalent and hexavalent forms of chromium exist in industrial wastewater,the toxicity caused by hexavalent chromium tends to be much higher than that by trivalent and; therefore, priority is given to the regulation of this pollutant at the point of discharge [2].
In order to reduce the level of Cr(VI) in these effluents to within the standard level, an efficient and low cost method needs to be developed. Various methods for the removal of Cr(VI) from industrial wastewaters include filtration, chemical precipitation,adsorption, electro-deposition and membrane systems, or even ion exchange process. Chemical precipitation and reduction processes require a further separation technique for the treatment and disposal of the high quantities of waste metal residual sludge they produce. These techniques require many treatment chemicals. The residual Cr(VI) concentration required in the treated wastewater can not be achieved due to the structure of the precipitates. The application of membrane systems for wastewater treatment is associated with major problems, such as membrane scaling, fouling and blocking. The disadvantage of the ion exchange process is the high cost of the resin, while the electro-deposition method requires more intensive energy than other methods [3]. To solve these problems, in recent years,investigations have been carried out for the effective removal of large quantities of Cr(VI) from wastewaters using low cost, nonconventional adsorbents, including Casurina leaves [4], leaf mould [5], moss pea [6], green algae [7], coconut waste [8] and rubber wood, which are economically viable [9]. Sawdust is a waste by-product of the timber industry, which is either used as cooking fuel or a packing material, but can also be economically used for the removal of heavy metals in wastewaters.
Hence, research on the application of holly sawdust as an alternative,low cost adsorbent for the removal of Cr in contaminated wastewaters is very promising. In this paper, the possibility of using holly sawdust to remove Cr from aqueous solution was investigated using batch adsorption studies. The effects of various factors, such as the initial pH and amount of adsorbent,on the removal efficiency of holly sawdust were also studied. Adsorption isotherm and kinetic studies were also investigated in order to understand the adsorption mechanism and efficiencies of holly sawdust. The adsorption isotherm data were also presented using the Langmuir and Freundlich models to describe the adsorption kinetics.
2.1. Preparation of Modified Holly Sawdust
Holly sawdust from a timber mill in Iran (Hamedan city) was washed with deionized water and dried in sunlight. To immobilize the color, the sawdust was modified with 1% formaldehyde,at a ratio of 1:5 (sawdust:formaldehyde, w/v) at 50˚C for 4 hr. The sawdust was filtered, washed with deionized water to remove free formaldehyde and activated at 80˚C in a hot air oven for 24 hr. One part of the modified sawdust was then mixed with one part concentrated sulfuric acid and heated in a oven for 24 hr at 150˚C. The heated material was washed with distilled water and soaked in 1% sodium bicarbonate solution overnight to neutralize any residual acid. The sawdust modified using sulfuric acid was dried in an oven at 105˚C for 24 hr, and then sieved in the size range of 70 mesh ASTM. The specific surface area (BET) of the modified sawdust was determined by the Research Institute of Petroleum Industry (RIPI) to be 365 m2/g.
2.2. Preparation of Cr(VI) Solution
A stock solution (1,000 mg/L) of Cr(VI) was prepared by dissolving a guaranteed reagent K2Cr2O7 into deionized water.Experimental solutions of the desired concentrations were obtained by successive dilutions. The initial solution pH was adjusted by the addition of 0.1 M NaOH or HCl. The experiments were carried out at room temperature (25 ± 2˚C).
An alkalimetric titration was used to characterize the surface acidity of the sludge sample according to the method of Huang and Morehart [10]. To prepare the samples for titration, a 1 g/L suspension was transferred into each of four beakers. Two were directly titrated, one with acid and the other with base (sample titrations) and the other two samples were titrated after removing the sample by passing through a 0.2 ㎛ filter (blank titrations).The initial pH value was measured, and the titrations of these solutions, under continuous mixing, were then performed using 0.1 M HCl to pH 2 or 0.1 M NaOH to pH 11. The volumes of acid/base added and the corresponding equilibrium pH values were recorded. The pH readings were taken every 30 min after each measured addition of acid or base. Pure N2 gas was continuously purged to exclude CO2. To obtain consistent titration data, the pH change after each acid/base addition was not allowed to exceed 0.3 units.
Plots for the net acid and net base titrations yielded intercepts and slopes from which the intrinsic acidity constants Ka1 int and Ka2 int, and the total number of surface sites were calculated. A zero point charge of pHzpc was also calculated from the intrinsic acidity constants.
The equilibrium time (3 hr) was determined from the kinetic data under the following conditions: pH = 7, adsorbent dosage= 6 g/L, initial Cr(VI) concentration = 60 mg/L. In each adsorption experiment, 100 mL of Cr(VI) solution, of known concentration and pH, was added to 0.6 g of modified sawdust in a 250 mL Erlenmeyer flask and mixed using a rotary mixer (H1-190M;Hanna Instruments, Kehl am Rhein, Germany(at 160 rpm for 3 hr. The aqueous samples were centrifuged) Sigma-301; Sigma-Aldrich, Osterrode am Harz, Germany) at 4,000 rpm for 10 min to remove the adsorbent and the Cr(VI) concentration was then analyzed. The residual Cr(VI) was analyzed using 1, 5-diphenylcarbazide and a UV spectrophotometer (HACH-DR 2500; HACH Co., Loveland, CO, USA) at a wavelength of 540 nm. The experiments were conducted with various amounts of the adsorbent(2 to 10 g/L), initial Cr(VI) concentrations (20 to 100 mg/L) and initial pHs from 2 to 12.
The surface acidity constants, pKa1 S = 3.37 and pKa2 S = 5.22,with a corresponding zero point charge of pHzpc = 4.3, were determined from the acidimetric-alkalimetric titrations. The total number of surface sites was calculated to be 8.15 × 10-4 mol/g.The site density of the sawdust was calculated to be 1.344 sites/nm2 using the specific surface area of 365 m2/g. The surface coordination sites consisted of inorganic surface hydroxyl groups.The surface acidity constants were composite values from the contributions of all protonated/deprotonated sites.
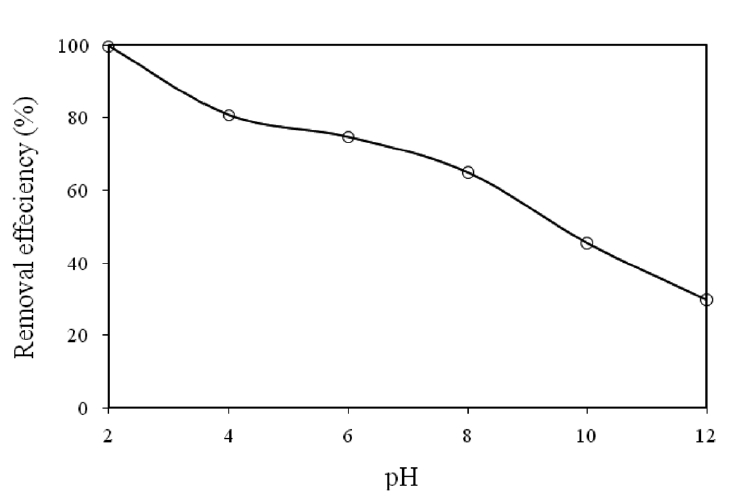
3.2. Effect of pH on Cr(VI) Removal
The effect of pH on the removal of Cr(VI) by modified holly sawdust was studied by changing the initial pH between 2 and 12. The relation between the initial pH of the solution and the
percentage Cr(VI) removed is shown in Fig. 1. The percentage removal of Cr(VI) decreased from 99.67 to 29.78% on increasing
the initial pH from 2 to 12. The favorable removal of Cr(VI) at a lower pH was related to both the anionic-type adsorption of Cr(VI) onto holly sawdust, as shown in Fig. 1, and the following acid-catalyzed reactions.
These results were considered since the dominant form of Cr(VI) is HCrO4? and the surface of the adsorbent is positively charged at low pH (pH = 2), but on increasing the pH, the HCrO4?species shifts to other forms; CrO4 2? and Cr2O7 2? . The decrease in the adsorption of Cr(VI) with increasing pH may have been due to the competition between the anions CrO4 2? and OH?. Similar observations have also been reported by other research groups[11, 12].
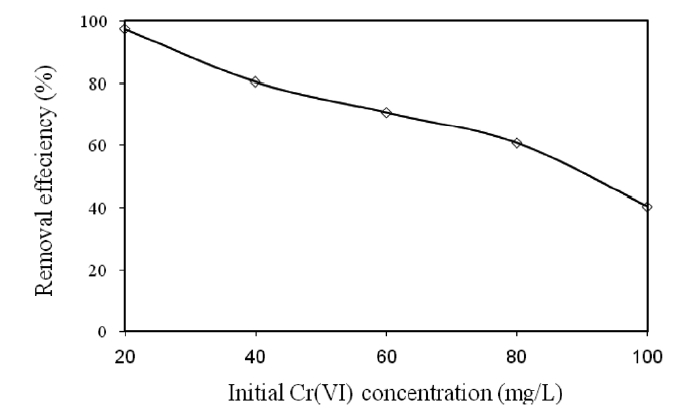
3.3. Effect of the Initial Cr(VI) Concentration on the Removal of Cr(VI)
Various concentrations (20, 40, 60, 80, and 100 mg/L) of Cr(VI) were used to investigate the effect of the initial Cr(VI)concentration on its removal by modified holly sawdust. The relationship between the initial Cr(VI) concentration of the solution and the percentage of Cr(VI) removed is shown in Fig. 2.Cr(VI) adsorption was significantly affected by the initial concentration of Cr(VI) in the aqueous solutions. The percentage of Cr(VI) removed decreased from 99.37 to 40.24% on increasing the initial Cr(VI) concentration from 20 to 100 mg/L. The decrease in the percentage removal can be explained by the fact the adsorbent had a limited number of active sites, which would have become saturated above a certain concentration.
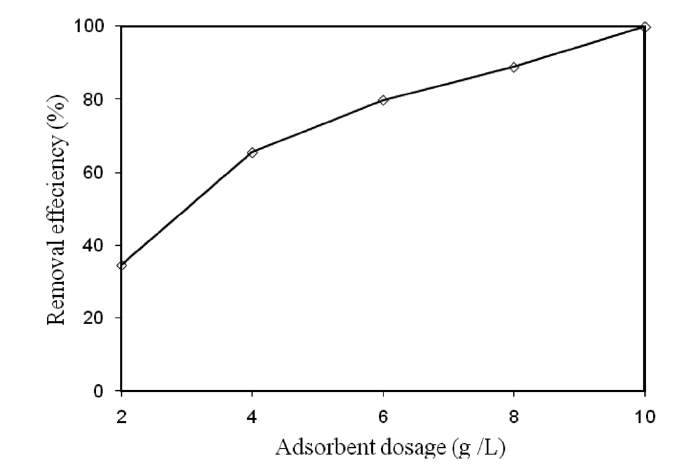
3.4. Effect of Adsorbent Dose on the Removal of Cr(VI)
The effect of the adsorbent dose on the removal of Cr(VI) by modified holly sawdust was studied by varying the adsorbent dose (2.0, 4.0, 6.0, 8.0, and 10 g/L). The relationship between the adsorbent dose and the percentage removal of Cr(VI) is shown in Fig. 3. The percentage removal of Cr(VI) increased from 34.65 to 99.99% on increasing the adsorbent dose from 2 to 10 g/L.The increased Cr(VI) removal on increasing the amount of sawdust was due to the increased surface area and adsorption sites available for adsorption. Similar observations have also been reported [13, 14].
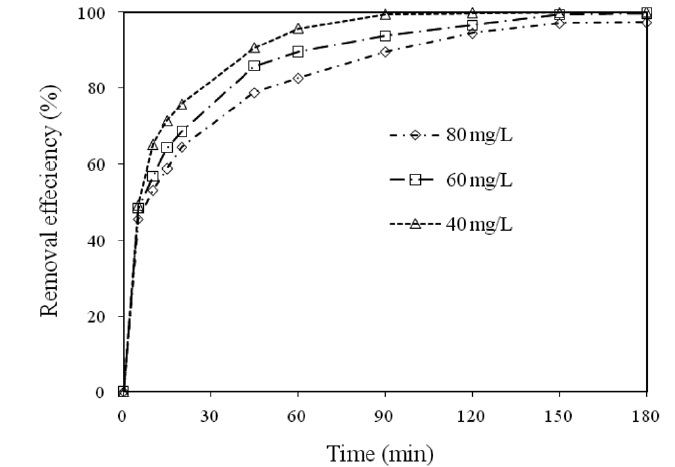
3.5. Effect of Contact Time on the Removal of Cr(VI)
The effect of the contact time on the removal of Cr(VI) by modified holly sawdust was studied by varying the contact time(5 to 180 min) for different initial Cr(VI) concentrations. The relationship between the contact time and the percentage removal of Cr(VI) is shown in Fig. 4. It was evident from Fig. 4 that time is an important adsorption parameter for the adsorption of Cr(VI)by the sawdust. On increasing the Cr(VI) concentration from 40 to 80 mg/L, the percentage removal decreased from 71 to 58%during the initial 15 min of contact time. Thereafter, the percentage removal of Cr(VI) slowly reached 99 and 97% for initial Cr(VI) concentrations of 40 and 80 mg/L, respectively, until 180 min. A further increase in the contact time had a negligible effect on the amount of Cr(VI) adsorbed. The percentage removal of Cr(VI) increased from 48.53 to 99.76% on increasing the contact time from 5 to 180 min for an initial Cr(VI) concentration of 60 mg/L. Fig. 4 shows that the optimal removal efficiency was reached within about 90 min. This probably resulted from the saturation of the adsorbent surfaces with Cr(VI), followed by the adsorption and desorption processes that occur after saturation.The rate of adsorption was decreased during later stages of the
Cr(VI) adsorption, probably due to the slow pore diffusion of the solute ions into the bulk of the adsorbent. Similar observations have also been reported in another investigation [15].
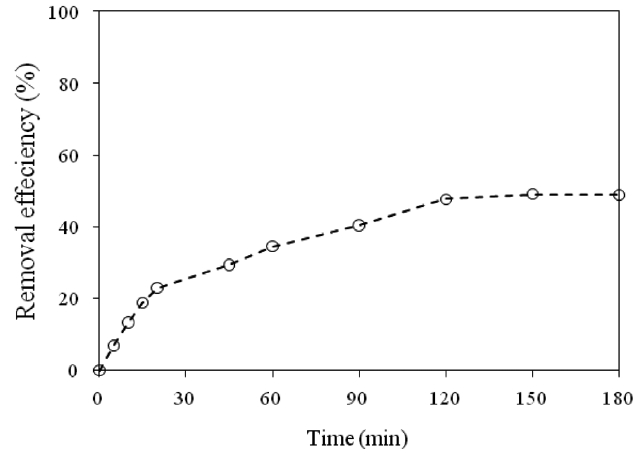
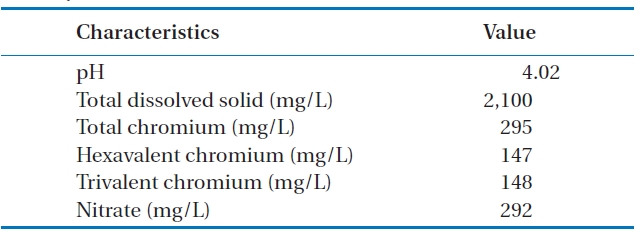
Fig. 5 shows the results for the treatment of a real wastewater.A removal efficiency of around 40% was reached after 90 min of contact time. In this case, since the concentration of total chromium was 295 mg/L, and other components also existing in the solution, the removal efficiency was high, as high as that of a unary system. The properties of the real wastewater are summarized in Table 1.
3.6. Adsorption Isotherm and Kinetics
Adsorption isotherm studies were conducted with an initial Cr(VI) concentration of 60 mg/L and adsorbent doses of 0.08, 0,1 0.15, 0.2, 0.25 3.0, 0.35, and 0.4 g/100 mL. After equilibration, the samples were separated and analyzed for their residual Cr(VI)concentrations. The equilibrium adsorption capacity was calculated using the following equation [5, 16, 17].
where qe (mg/g) is the equilibrium adsorption capacity, C0 and Ce the initial and equilibrium concentrations (mg/L) of Cr(VI),respectively. V (L) is the volume of solution and M (g) the weight of the adsorbent.
For modeling of the equilibrium data, both the Langmuir and Freundlich isotherms were applied.
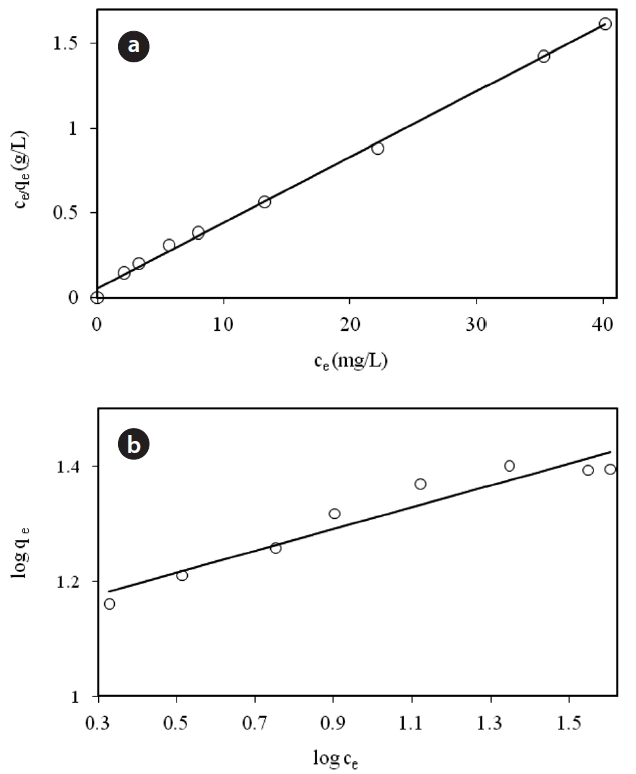
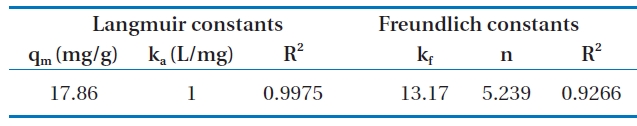
Fig. 6 shows the linear plot of Ce/qe versus Ce. The calculated values of qm and ka are presented in Table 2. The value of the correlation coefficient (R2 = 0.9975) indicated good agreement between the experimental equilibrium data and the Langmuir isotherm. The monolayer saturation capacity was 17.857 mg/g.The applicability of the Freundlich sorption isotherm was also analyzed by plotting log(qe) versus log(ce), but no good agreement was observed in this case (Fig. 7). Table 2 also shows the Freundlich sorption isotherm constant and the correlation coefficients.
The adsorption kinetics describes the rate of uptake of chromium ions onto the modified holly sawdust. Kinetics studies
[Table 1.] Characteristics of a real electroplating wastewater in Hamadancity
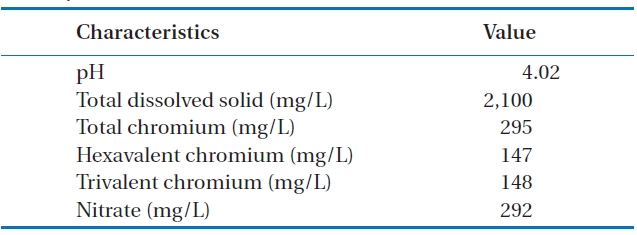
Characteristics of a real electroplating wastewater in Hamadancity
[Table 2.] Isotherm constants for the adsorption of Cr(VI) onto modifiedholly sawdust
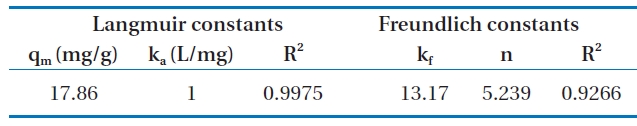
Isotherm constants for the adsorption of Cr(VI) onto modifiedholly sawdust
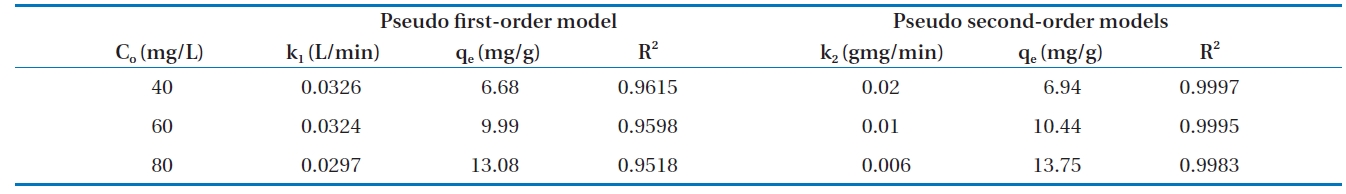
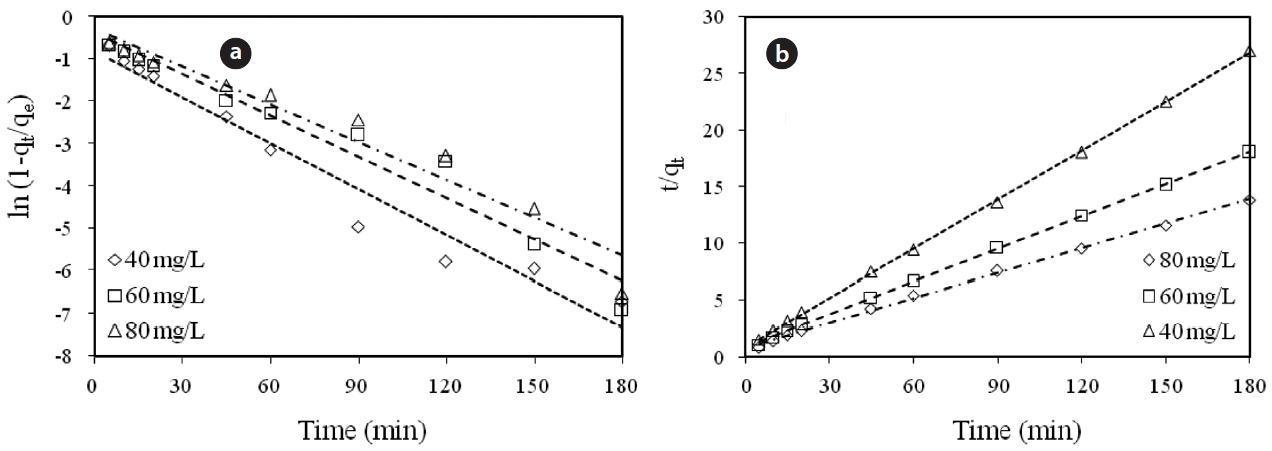
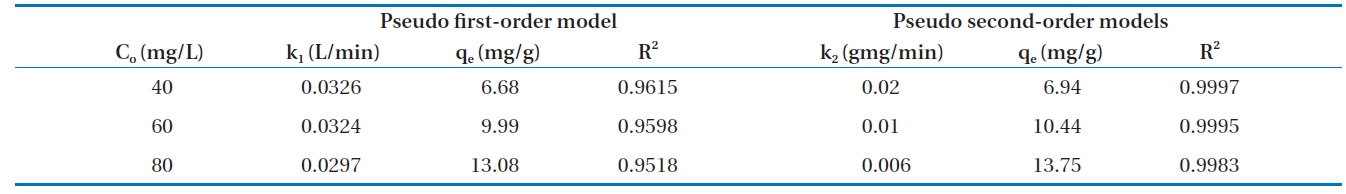
Calculated kinetic parameters for the pseudo first-order and pseudo second-order models for the removal of Cr(VI) by modified hollysawdust
adwere conducted in a series 250 mL Erlenmeyer flasks filled with 100 mL of solutions with initial Cr(VI) concentrations of 40, 60,and 80 mg/L and an adsorbent dose of 0.6 g/100 mL, at pH 7 ±0.2. At specified time intervals, the samples were separated and analyzed for their residual Cr(VI) concentrations. This study revealed that 60 to 80% of the adsorption took place within the first minute of contact. The kinetics for the adsorption of Cr(VI)onto the modified holly sawdust was analyzed using pseudo first-order and pseudo second-order models [18] at various initial Cr(VI) concentrations.
Fig. 7 shows linear plots of the pseudo first-order (a) and pseudo second-order (b) models for the removal of Cr(VI) by the modified holly sawdust. The obtained values of k1, k2 and qe are summarized in Table 3. The value of correlation coefficient (R2= 0.999) indicated good agreement between the kinetic data for the adsorption of Cr(VI) onto the modified holly sawdust and the pseudo second-order model.
The major findings of this study were as follow:
1) The removal of hexavalent chromium from aqueous solutions increased with increasing adsorbent dosage and contact time, suggesting the dependence of the efficiency on the amount of surface sites of the adsorbent.
2) The experimental results indicated that the pH of the aqueous solution was crucial to the adsorption of Cr(VI), possibly because the presence of various Cr(VI) species and the surface charges on the adsorbent are highly pH dependent. The results showed that the removal of hexavalent chromium from aqueous solutions by modified holly sawdust was effective for solution pHs below 6.
3) The experimental results were analyzed using the Langmuir and Freundlich equations. The correlation coefficient for the Langmuir isotherm was significantly better than that for the Freundlich isotherm.
4) The adsorption kinetics of hexavalent chromium onto modified holly sawdust was analyzed using pseudo first-order and pseudo second-order models. The pseudo second-order model better explained the kinetic of hexavalent chromium adsorption.
5) Finally, the results showed that the modified holly sawdust can be used as a low cost adsorbent for the treatment of aqueous solutions containing chromium.