The equilibrium and kinetic adsorption of arsenic on six different adsorbents were investigated with one synthetic and four natural types (two surface and two ground) of water. The adsorbents tested included magnetic ion exchange resins (MIEX), hydrous ion oxide particles (HIOPs), granular ferric hydroxide (GFH), activated alumina (AA), sulfur modified iron (SMI), and iron oxide-coated microsand(IOC-M), which have different physicochemical properties (shape, charge, surface area, size, and metal content). The results showed that adsorption equilibriums were achieved within a contact period of 20 min. The optimal doses of adsorbents determined for a given equilibrium concentration of Ceq = 10 μg/L were 500 mg/L for AA and GFH, 520?1,300 mg/L for MIEX, 1,200 mg/L for HIOPs,2,500 mg/L for SMI, and 7,500 mg/L for IOC-M at a contact time of 60 min. At these optimal doses, the rate constants of the adsorbents were 3.9, 2.6, 2.5, 1.9, 1.8, and 1.6 1/hr for HIOPs, AA, GFH, MIEX, SMI, and IOC-M, respectively. The presence of silicate significantly reduced the arsenic removal efficiency of HIOPs, AA, and GFH, presumably due to the decrease in chemical binding affinity of arsenic in the presence of silicate. Additional experiments with natural types of water showed that, with the exception of IOC-M, the adsorbents had lower adsorption capacities in ground water than with surface and deionized water, in which the adsorption capacities decreased by approximately 60?95%.
Arsenic contamination in natural water is a global problem,and arsenic pollution has been reported in many areas of the world. The US Environmental Protection Agency set the arsenic standard for drinking water at 10 parts per billion (10 μg/L) in 2006 [1]. Various studies have investigated arsenic removal using different technologies, including oxidation [2, 3], alum/iron coagulation [2, 4, 5], sorption/ion-exchange (activated alumina,iron coated sand, and ion-exchange resin) [6, 7], and membrane filtration [8, 9]. Sorption techniques are relatively simple to conduct and are cost effective. Various materials have already been tested for their sorption efficiency of arsenic from water. The wide variety of different adsorbents ranges from natural materials to specially designed technical particles.
Magnetic ion exchange resins (MIEX) developed by Orica Watercare are ion-exchange resin particles impregnated with a magnetized component within their structure. The pore size of these anion-exchange resins is 2?5 times smaller than that of conventional resins. Resins with small pores exhibit high rates of exchange, because they have a large external specific surface area [10]. Sharma et al. (2009) reported that magnetic nanoparticles are effective for removing metals such as chromium, zinc,and arsenic. The adsorption of arsenite and/or arsenate has been studied for amorphous and/or hydrous ion oxide particles(HIOPs) prepared by neutralizing FeCl3 solution by adding NaOH[11-14]. Granular ferric hydroxide (GFH) is a granular adsorbent developed by the Technical University of Berlin, Germany [15]; it is prepared by neutralizing and precipitating FeCl3 solution with sodium hydroxide. GFH was investigated for use as a potential adsorbent for arsenic removal from natural water in Germany[15] and was shown to be very effective for arsenate removal [6, 16]. Other materials such as activated alumina (AA) have also shown good results (uptake capacity up to 11 mg/g) [17]. In the past, AA was the most commonly used adsorbent for arsenic removal and is also relatively inexpensive. Sulfur-modified iron(SMI) is an adsorptive medium recently developed by Santina& Thompson (Concord, CA, USA). The medium is composed of zero-valent iron (sponge iron) and elemental sulfur compounds.SMI has a potentially redox reactive surface, which is capable of removing both As(III) and As(V) species. The removal of As(V)by SMI is pH dependent, and maximum removal of arsenic is observed in the acidic pH range [18, 19]. Iron oxide-coated microsand(IOC-M) prepared in the lab shows effective removal of uncomplexed and ammonia-complexed cationic metals (Cu,Cd, Pb, Ni, and Zn), as well as some oxyanionic metals (SeO3 and AsO3) from simulated and actual water streams over a wide range of metal concentrations [20].
Previous studies have shown that arsenic removal depends on water chemistry and adsorbent characteristics. However,these studies were limited to a few adsorbents and/or included only a few physicochemical properties to address removal mechanisms from synthetic types of water. Therefore, the objective of this study was to determine the physicochemical processes related to the removal of arsenic from contaminated water under the defined conditions of both synthetic and natural water using six adsorbents. The adsorbents evaluated at this bench-scale level included MIEX, HIOPs, AA, GFH, SMI, and IOC-M. The approach was to perform a detailed characterization of these adsorbent materials in terms of their physicochemical properties(i.e., to determine their dry weight, metal content, particle size distribution, surface area, crystalline structure, surface charge,and texture) and then evaluate their potential for arsenic removal with a wide range of removal mechanisms over a wide pH range and at different background anion concentrations.
2.1.1. MIEX
MIEX resins were obtained from Orica Watercare (Adelaide,Australia). Unlike other ion-exchange resins, MIEX does not require pre-treatment for solids removal. MIEX was stored as a slurry and administrated volumetrically (i.e., mL/L that was later converted to a weight basis [mg/L] using dry weight). MIEX was regenerated using NaCl and reused repeatedly with a small loss of capacity. Aliquots of MIEX were oven-dried; various measurements were then taken as described in Table 1.
2.1.2. HIOPs
HIOPs were produced in the lab by neutralizing a FeCl3 solution by adding NaOH. The solution was then rinsed with deionized(DI) water to remove chloride and heated to 110°C for 1 to 14-hr [21]. The resulting solid was non-compressible and semi-crystalline in structure. X-ray diffraction (XRD) analysis of the media indicated the presence of various types of iron oxides,including hematite, geothite, and iron oxide hydrate. Similar to MIEX, HIOPs was stored in solution and administered volumetrically(i.e., mL/L). Doses are reported on a weight-basis by their known titer (i.e., mg/mL).
2.1.3. GFH
GFH was prepared in the lab by neutralizing and precipitating a FeCl3 solution with NaOH. The ferric hydroxide precipitate was washed several times with demineralized water and then centrifuged and granulated using high pressure (for dewatering)to form a crystallized medium. The medium contained about 50% water and was very porous (75% porosity), with large inner tunnels to accommodate anions or contaminants.
2.1.4. AA
AA (a white, porous, crystalline, and granular substance)was obtained from Alcoa (Pittsburgh, PA, USA). The AA used in the adsorption studies was between 100 and 140 mesh in size(0.106?0.125 mm). A stock solution of 0.5 g AA/mL was prepared by adding 40 g of AA to DI water to produce a total solution volume of 80 mL. The pH of the solution was then adjusted to 7.0 by adding 2 N HCl.
[Table 1.] Characteristics of various adsorbents used in this study
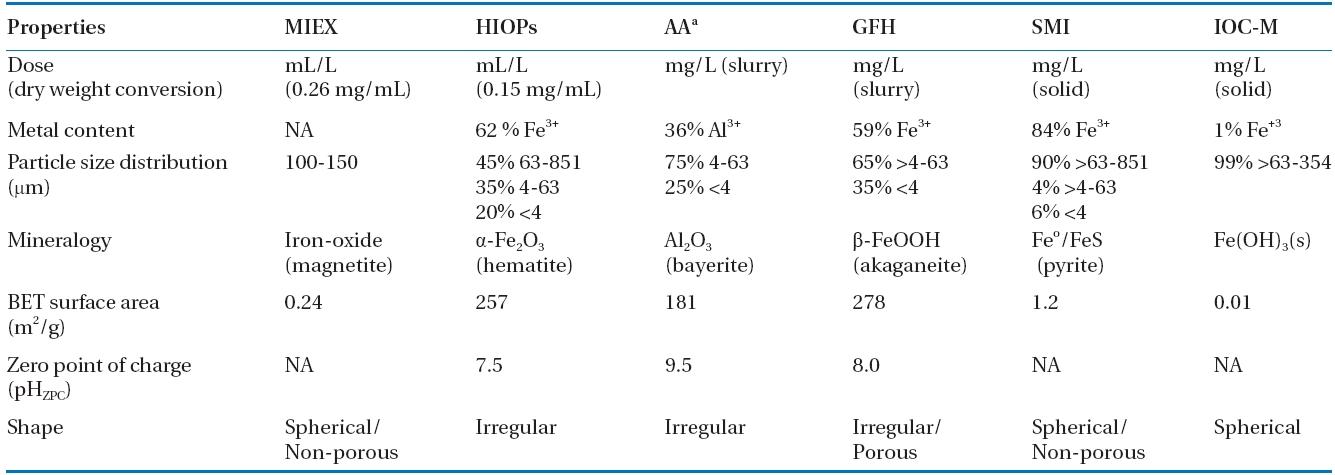
Characteristics of various adsorbents used in this study
2.1.5. SMI
SMI developed by Santina & Thompson was prepared. To prepare the SMI, iron and sulfur were mixed together, which produced an exothermic reaction. The reaction was allowed to continue until no further heat was detected, at which point the media was air-dried and sieved.
2.1.6. IOC-M
IOC-M was prepared in the lab based on the methods of Benjamin et al. (1996). Pre-washed microsand (100-mesh size;0.1?0.15 mm) was rinsed with DI water and soaked in a 50%H2SO4 solution for 24-hr, rinsed until the pH exceeded 5.0, and then dried at 110°C for 20-hr. The washed microsand was then stored in a polystyrene bottle. The iron coating solution was prepared by mixing 100 g FeCl3 in 100 mL of DI water (0.37 M Fe+3)and then adding 0.6 mL 10 M NaOH to yield a Fe:OH ratio of 1.0:0.037. The cleaned microsand (200 mL) was placed in a glass heat-resistance beaker along with 80 mL of coating solution (1.6 g as Fe); this was then baked at 110°C overnight. The sand was broken, filtered through a 100 mesh sieve (<0.1 mm), put back into the oven for an additional 5-hr, and stored prior to use.
The As(V) stock solution was prepared with Na2HAsO4·7H2O,and then 500 mL of an arsenic solution (1,000 μg/L) was prepared by mixing DI water and the stock solution. Isotherm experiments were started by adding a constant amount of adsorbent.Applied adsorbent doses ranged from 0 to 7,500 mg/L.Kinetic experiments were conducted to determine dose and contact time requirements with contact times of 0, 2, 10, 30, and 60 min. The isotherm experiments were conducted at two different pH values (pH 5.5 and 7.5) for MIEX, HIOPs, AA, GFH, and SMI and at pH 4.0 and 7.0 for IOC-M. For the pH 7.0 and 7.5 experiments, 1 mM of organic buffer (N,N-bis (2-hydroxyethyl)-2-aminoethanesulfonic acid) was used to avoid interference with arsenic, as established in control experiments. Samples of all adsorbents were collected after mixing for 1-hr (much longer than the previously determined equilibrium times) to ensure complete equilibrium. Details on the experimental conditions are summarized in Table 2. All work performed with DI water was to avoid matrix effects from the presence of other contaminants.Dose and contact time requirements were determined by adding varying amounts of adsorbent introduced on a v/v basis(mL/L) for MIEX and HIOPs and a w/v basis (mg/L) for GFH,SMI, AA, and IOC-M.
Several additional experiments with the adsorbents, excluding IOC-M, were conducted to remove arsenic from two surface water samples and two ground water samples spiked to an arsenic concentration of 100 μg As(V)/L. The adsorbent dosages used were those previously determined in kinetic studies. The natural water samples were obtained from the Los Angeles Aquaduct Filtration Plant (LAAFP; Los Angeles, CA), Billings, MT for the surface water, and the cities Phoenix and Tucson, AZ for the ground water. These samples were collected in four 20-L carboys,shipped overnight, and stored at 4°C prior to experimentation.Table 3 lists the water quality parameters, including major ions and arsenic species, for the natural water as received. The anions and cations in the water were measured to assess how the co- and counter ions would influence arsenic removal. The ground water had a higher concentration of ions when compared to that of the surface water. All of the water naturally contained<12 μg/L As(V) and <1 μg/L As(III), with the exception of the Phoenix ground water, which contained 7.3 μg /L As(III).The mixture was stirred at 150 rpm for a period of time equal to or greater than the determined reaction equilibrium time. Samples were collected at various time intervals, and the adsorbent was immediately removed by either centrifugation or filtration through a 0.45- ㎛ membrane prior to arsenic analysis.
Arsenic was analyzed based on Standard Method 3120B [22]using an inductively coupled plasma (ICP) mass spectrometer(UltraMass 700 or Liberty Series II; Varian Inc., Palo Alto,CA, USA) with a minimum detection limit of 0.5 μg/L. Surface charge was determined by zero point of charge (pHZPC) measurements using a zeta potential analyzer (ZetaPlus; Brookhaven Instruments, Holtsville, NY, USA) at pHs 3.5?9.5. Aliquots of the various media were spiked into 300 to 1,200 μS/cm ionic strength solutions provided by KCl, and pH was controlled by adding HCl and/or KOH. A turbidimeter (model 2100N; Hach
[Table 2.] Conditions for the kinetics study of various adsorbents with deionized water
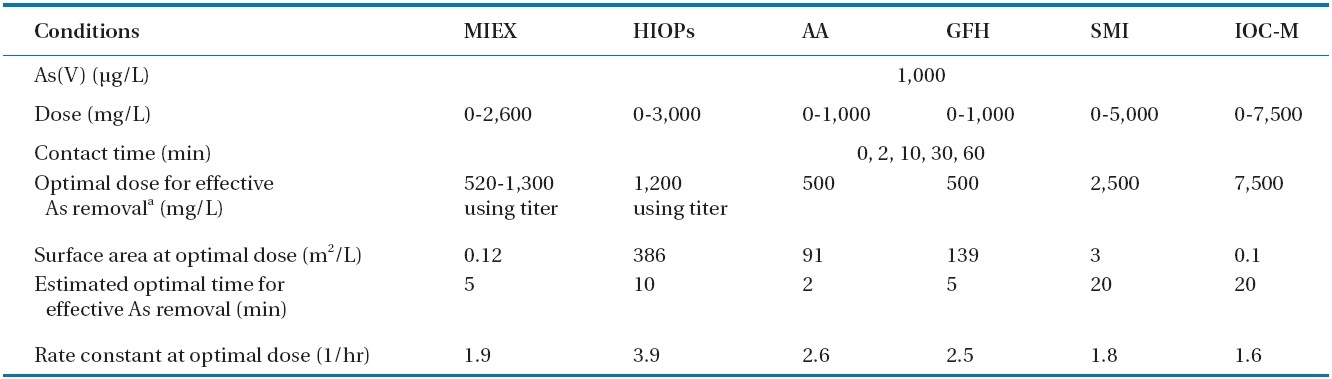
Conditions for the kinetics study of various adsorbents with deionized water
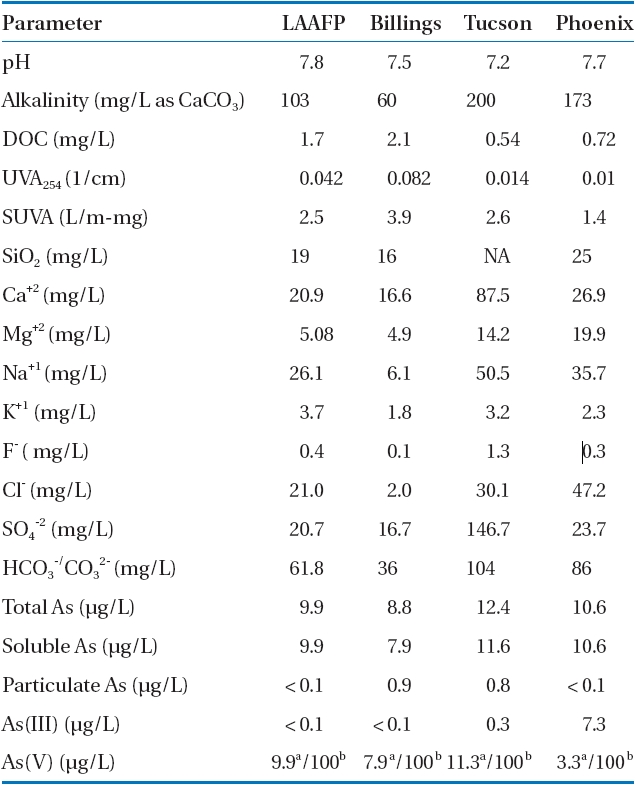
Water quality compositions of surface (LAAFP CA andBillings MT) and ground (Tucson and Phoenix AZ) water samples
Co., Loveland, CO, USA) was used to measure turbidity based on Standard Method 2130 [22]. Total alkalinity was determined by titration methods based on Standard Method 2320B [22]. Anion analysis was conducted by ion chromatography (DX300; Dionex Co., Sunnyvale, CA, USA) with an AS9-HC column (Dionex) used for the major anion measurements. Cations were measured with an ICP emission spectroscopy instrument (Liberty-Series II; Varian,Palo Alto, CA, USA). A DS-130 (AKASHI, Tokyo, Japan) scanning electron microscope (SEM) was employed to determine the morphology of the tested adsorbents. The particle size distribution was determined using a particle size analyzer (Malvern Mastersizer 2000; Malvern Instruments, Worcestershire, UK). Xray diffraction (Scintag Pad5) was used to characterize the adsorbents.
3.1. Adsorbent Characterization
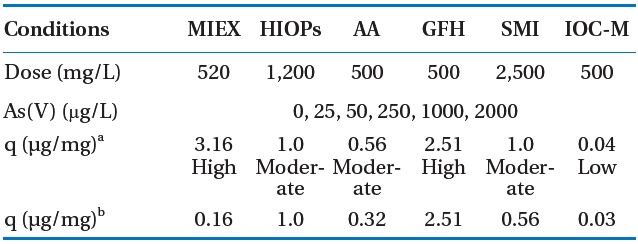
A physicochemical characterization was performed to determine the metal content, particle size distribution, shape, mineralogy,surface area, and charge of the tested adsorbents (Table 1). The adsorbents had different metal content: SMI (84% Fe3+)> HIOPs (62% Fe3+) > GFH (59% Fe3+) > IOC-M (<1% Fe+3), and 36% Al3+ for AA. HIOPs, AA, and GFH had relatively smaller particle sizes than those of the other adsorbents (MIEX, SMI, and IOC-M). Additionally, these larger media had non-porous structures and, thus, significantly small surface areas, so it was assumed that arsenic was removed predominantly on the media surface. Mineralogy determined by XRD indicated the presence of metal oxides; predominantly hematite from HIOPs, bayerite from AA, iron oxide hydrate (i.e., akaganeite) from GFH, and pyrite from SMI. The average measured Brunauer?Emmett?Teller surface areas of the adsorbent media were >181 m2/g for GFH> HIOPs > AA and <1.2 m2/g for SMI > MIEX > IOC-M. Surface charge measurements indicated that all adsorbents were positively charged for effective arsenate removal at neutral or lower pH conditions. Finally, texture determinations by SEM revealed that some of these adsorbents were more regular and spherical in shape (MIEX, SMI, and IOC-M) than others (HIOPs, AA, and GFH). Moreover, SEM also provided information regarding their porosity; GFH was more porous than MIEX and SMI, suggesting that GFH benefits from inner pore adsorption/diffusion.
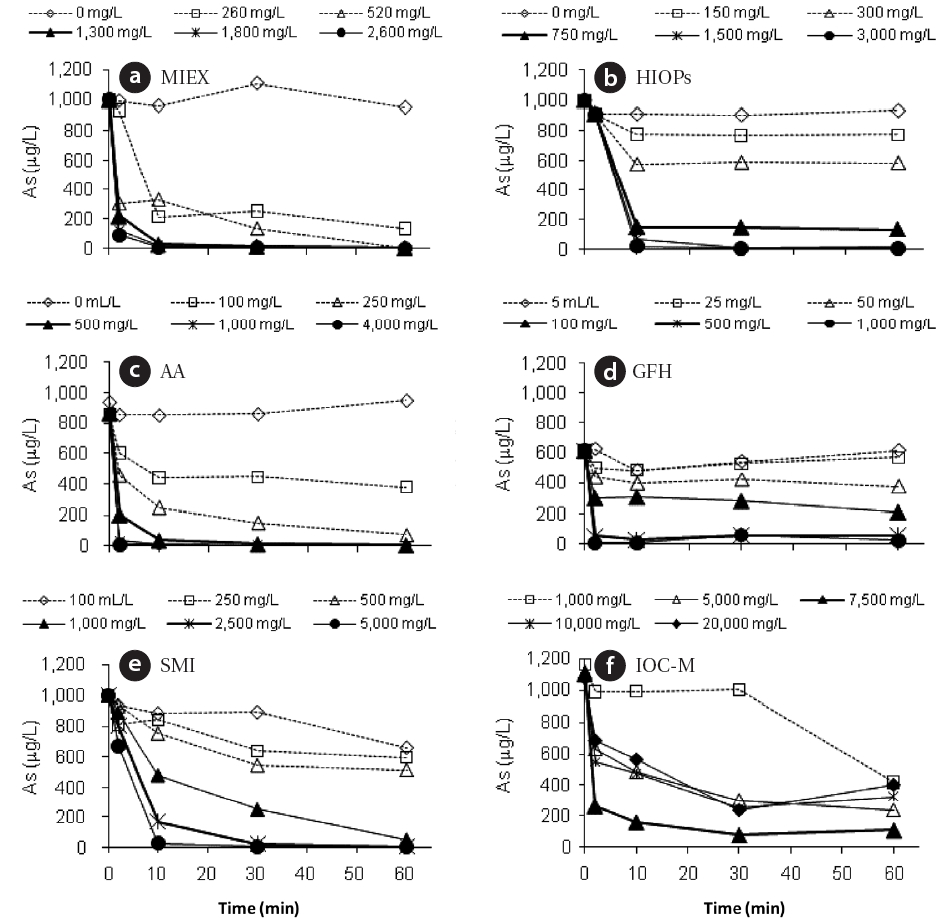
All six adsorbents were tested following an identical procedure using 1,000 μg/L As(V) in a synthetic solution containing no background co-ions and natural organic matter. To assess the performance of the different materials, batch experiments were conducted at pH 5.5 by adding variable amounts of adsorbents on a volume or weight basis, as shown in Table 2. The pH selected minimized the need for pH control and buffering during the experiment. The two main parameters controlling the efficiency of these adsorbents were contact time and sorption capacity (dictated by the amount/dose requirement in the contactor). Therefore, a series of tests was performed with synthetic water to estimate these two important parameters. Fig. 1 shows the arsenic removal profiles for the various adsorbents in relation to time and dose for all six adsorbents. The high arsenic concentration selected for these experiments provided the greatest analytical sensitivity for determining the effectiveness of the media to remove arsenic from solution. These results show that adsorption equilibriums (10 μg/L) for the adsorbents were achieved within a contact period as short as 2 min for AA,approximately 5 min for MIEX and GFH, 10 min for HIOPs, and as long as 20 min for SMI and IOC-M (Table 2). The experiments also indicated that the optimal dose of adsorbent for treating the 1,000 μg/L arsenic solution varied depending on the adsorbent;approximately 500 mg/L for AA and GFH, <1,300 mg/L for MIEX and HIOPs, 2,500 mg/L for SMI, and 7,500 mg/L for IOCM.MIEX, HIOPs, AA, and GFH adsorption rates accelerated with an increase in the amount of adsorbent added, whereas adsorption rates of SMI and IOC-M remained constant with increasing adsorbent dosages. Rapid kinetics have significant practical importance, as they allow for a shorter media contact time for a given water type, thereby reducing the need for a higher media dose. Based on these results, MIEX was very effective as an adsorbent with a rapid adsorption rate and capacity. Comparing all of the adsorbents on a weight basis in DI water, the effectiveness for removing As(V) was in the following order: MIEX > GFH > AA> HIOPs > SMI > IOC-M.
Arsenic removal by adsorbents is somewhat complex. However,potential removal mechanisms can be assumed based on the batch experiment results and absorbent characteristics.For MIEX, the primary mechanism for arsenic removal is by ion exchange, whereas negatively charged As(V) (as HAsO42- or H2AsO4-) is removed by exchanging Cl-. For ion oxide particles
such as HIOPs, the primary mechanism for arsenate removal is surface complexation adsorption onto hydrous iron oxide surfaces[23-25]. For GFH and AA, the primary mechanism may be site-specific adsorption on the adsorbent surfaces and on the inner pores of the media. One hypothesized mechanism for arsenic removal by SMI is via reduction and surface precipitation,in which zero-valent iron (Fe0) oxidizes to Fe3+ while reducing arsenate to As-. The primary mechanism postulated for arsenate removal by IOC-M is assumed to be surface adsorption onto the iron-oxide sand coating.
3.3.1. Effect of pH
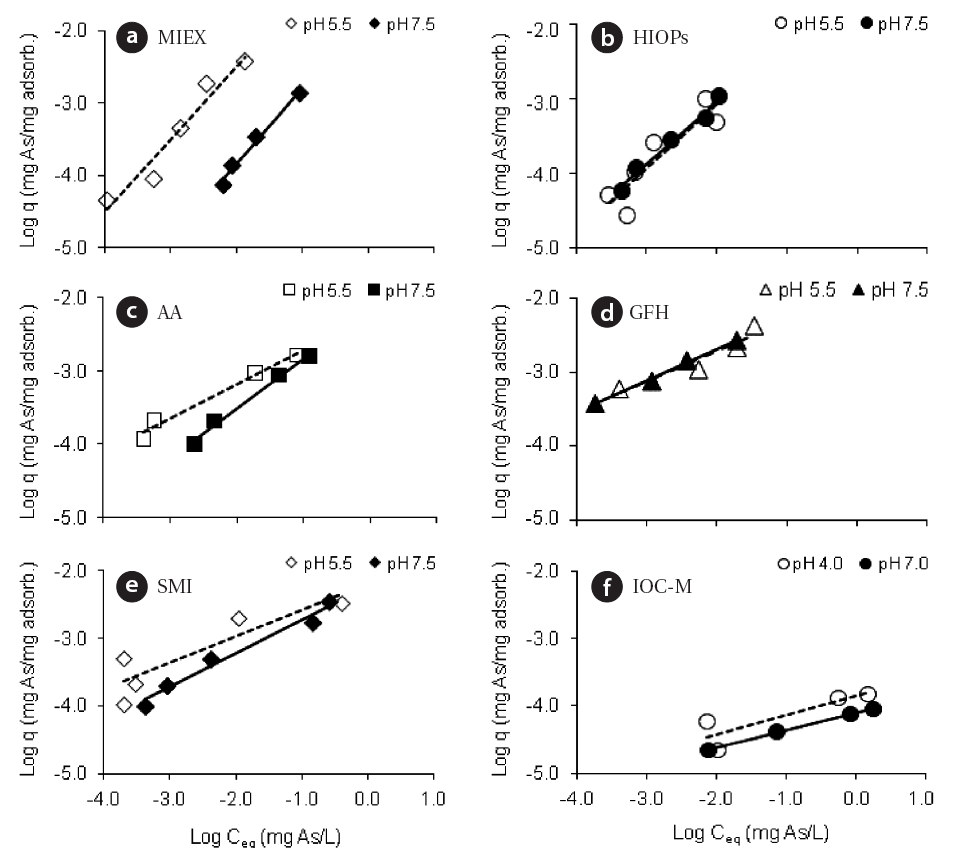
After the adsorption kinetics tests, experiments were performed to develop adsorption isotherms in DI water based on various adsorbent concentrations. The isotherms were developed at two different pH values (pH 5.5 and 7.5) for all adsorbents excluding IOC-M (pH 4.0 and 7.0). For the pH 7.5 tests,an organic buffer (C6H15NO5S, N,N-bis (2-hydroxyethyl)-2-aminoethanesulfonic acid) was used to avoid interference with arsenic. Samples of all adsorbents were collected after 1-hr of mixing. The Freundlich equation was used to produce the loglog linear isotherms shown in Fig. 2. The figure indicates that higher pH conditions provide significantly lower sorption capacities for every medium except HIOPs and GFH. HIOPs and GFH had nearly identical adsorption capacities at both pH 5.5 and 7.5, indicating that the use of these two media may be more applicable over a wider range of pHs in natural water without diminished adsorption capacities. The results also showed that at lower pH MIEX tended to have a higher adsorptive capacity for arsenic. The reason for the increased adsorption at pH 5.5 can be attributed to increasing cationic charge density of the resin and/or a better exchange at lower pH conditions, at which monovalent H2AsO4- dominates. Both the kinetics and isotherm results indicate that MIEX tended to be very fast and effective as an adsorbent for arsenate removal; adsorption occurred within its surface by ion-exchange mechanisms, in which the chloride ion within the resin structure was preferentially exchanged with monovalent arsenate species.
Table 4 summarizes the testing results and compares adsorptive capacities of the adsorbents for a given equilibrium concentration of Ceq = 10 μg/L As with DI water. GFH and HIOPs did not have significantly decreased adsorption capacities when the pH was increased from 5.5 to 7.5. The results showed that GFH and HIOPs had substantially higher arsenic adsorption capacities than those of the other adsorbents at both pH 5.5 and 7.5,whereas IOC-M adsorption capacities were at least an order of
[Table 4.] Conditions for adsorption isotherms of various adsorbentswith deionized water
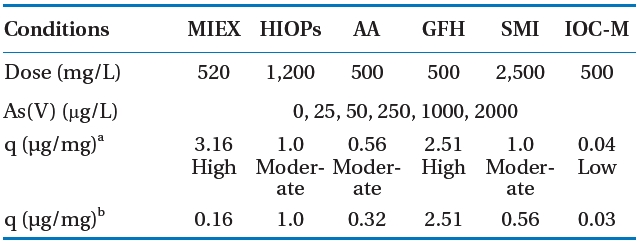
Conditions for adsorption isotherms of various adsorbentswith deionized water
magnitude less than the other adsorbents at both pH conditions.However, it should be noted that IOC-M had only approximately 2% of the Fe compared to that of GFH or HIOPs. Due to these results, IOC-M was not included in subsequent experiments.
3.3.2. Effect of background ions
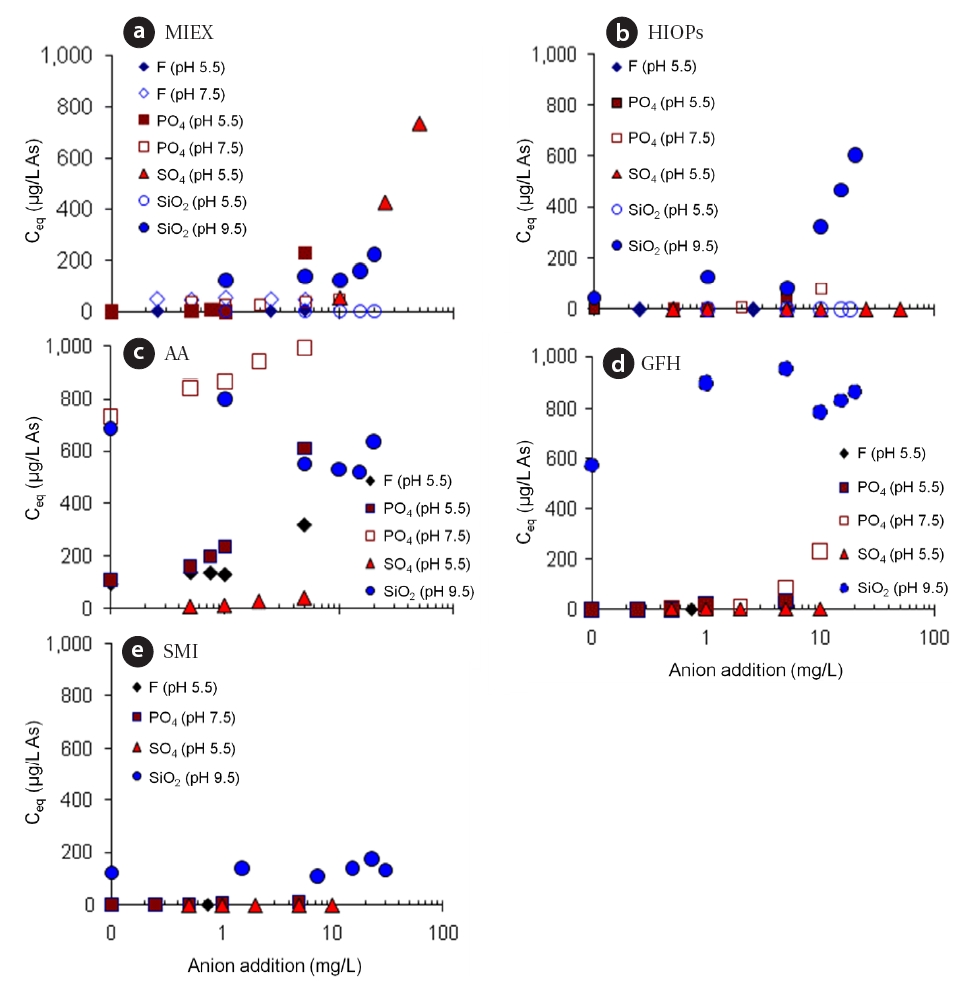
Various anions are present in natural waters and have an effect on arsenic adsorption due to competition for reaction sites.Arsenate removal is substantially reduced by competition from anions (sulfate, phosphate, silicates, and fluoride) and/or NOM(i.e., fulvic and humic acids) [26-29]. Several experiments were performed in DI water involving adding various amounts of competing anions to 1,000 μg/L As(V) at the optimal adsorbent dosages of all adsorbents, excluding IOC-M. The competing anions used were fluoride, phosphate, sulfate, and silicate at various concentrations, ranging from 0 to 50 mg/L, and at different pH conditions (5.5, 7.5, and/or 9.5). The higher pH was used to quantify the effects of SiO32-, whose form is predominant only when the pH is >9.42 (i.e., pKa of silica, [30]). For the pH 7.5 and 9.5 tests, an organic buffer (BES) was used to avoid interference with arsenic. Samples of all adsorbents were collected after 1-h of mixing.
Fig. 3 shows the remaining concentrations of arsenic after single additions of target ions. No anion had similar impacts on all adsorbents. However, it appeared that the effect of sulfate on arsenic removal by any of the iron media (HIOPs, GFH, and SMI)was minimal. A previous study with ferric chloride showed that the removal of As(V) and As(III) was not affected in a pH range of 4 to 10 in the presence of sulfate (0?300 mg/L) [27]. These results suggest that the sulfate binding affinity for ferric hydroxide was much weaker than As(V) and As(III). Although sulfate significantly inhibited arsenic adsorption on MIEX, MIEX was the only media that had arsenic adsorption inhibited by the presence of sulfate. This result conforms with existing information that sulfate is preferentially sorbed onto ion-exchange resin sites
over arsenic [31]. Fluoride had no effect on MIEX or the iron media,whereas the presence of fluoride (<5.0 mg/L) considerably reduced the efficiency of As(V) removal by AA (approximately<35%). The effect of phosphate (<5 mg/L) on arsenic removal was relatively low for MIEX, HIOPs, GFH, and SMI compared to AA. Clearly, arsenic removal by AA decreased significantly with an increase in phosphate concentration (<5 mg/L), in which the remaining arsenic concentrations were approximately 600 μg/L at pH 5.5 and 1,000 μg/L at pH 7.5 (almost no arsenic removal).This was presumably due to the decrease in the chemical binding affinity of arsenic in the presence of phosphate at the higher pH level. A previous study showed that the uptake of arsenate to AA remained almost constant for a pH <6, and then dropped significantly under higher pH conditions [17]. For pH 5.5, the surface of AA was predominantly positively (pHIEP = ~9.0), and the major arsenic species was H2AsO-. Therefore, as pH increased,the portion of positively charged surface sites on AA decreased,causing a reduction in the adsorption uptake. For GFH, no significant reduction in arsenic removal was observed in the presence of those ions excluding silicate at pH 9.5.
Previous studies have indicated that the presence of silicate significantly reduces the efficiency of arsenic removal by iron(II)/iron (III) [28] and ferric chloride [27]. In the presence of silicate,the soluble iron concentration increases significantly in the high pH range (>8.6) [27]. In that study, when the silicate concentration was 5 mg/L Si, and the pH was increased from 8.6 to 9.4, the soluble iron concentration increased from 47 to 2,040μg/L. When the pH was between 4 and 8.6, more than 97% of the iron formed hydroxide precipitates in the silicate solution [27].In our study, it was assumed that the soluble ion and aluminum concentrations increased in the presence of silicate (<20 mg/L),which could cause significant reductions in arsenic removal at pH 9.5 for HIOPs, AA, and GFH. Overall, MIEX was the most effective in terms of adsorptive capacity, but it was also the most sensitive to the presence of various anions. The results indicate that although MIEX outperformed most of the other adsorbents for arsenic removal in anion-free water, it may not be as effective in natural water due to the presence of other anions. The adsorptive capacities of adsorbents such as SMI, GFH, and HIOPs could be less affected by the presence of various anions as compared to those of MIEX and AA.
3.3.3. Comparison of adsorbents in natural waters
The adsorbents, excluding IOC-M, were evaluated for their
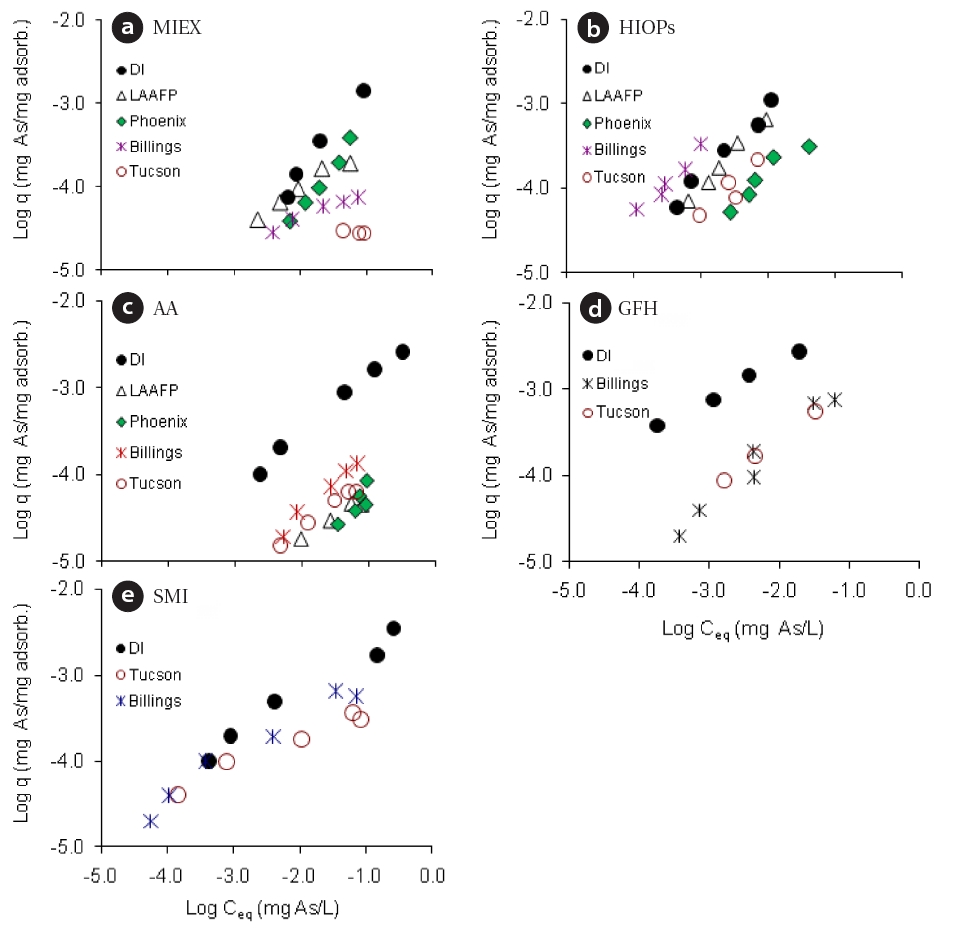
ability to remove arsenic from a spiked (100 μg As(V)/L) surface(LAAFP, CA, and Billings, MT, USA) and ground (Phoenix and Tucson, AZ, USA) water samples with similar pH levels (pH 7.2?7.8) and synthetic water (pH 7.5). The natural water samples contained various ions such as chloride, sulfate, fluoride, carbonate,and silica at different concentrations (Table 3). These differences could significantly affect the adsorbents by reducing their adsorptive capacities. Fig. 4 shows adsorption isotherms for the media in both DI and the natural water samples at a contact time of 60 min. The figure clearly shows that each medium had reduced adsorption capacities in the natural water samples,and that AA and GFH exhibited the most significant decreases.The reduction of adsorptive capacity in the natural water samples were a result of multiple competing anions in the water. The one exception was the performance of HIOPs in the Billings water;for a given equilibrium concentration of Ceq = 10 μg/L As, the HIOPs adsorption capacities (1.0 μg/mg for both DI and Billings waters) did not decrease, whereas the capabilities of the other media decreased by 42?88%. In general, the five media had lower adsorption capacities in the ground water samples (Tucson and Phoenix) than in the surface and DI water samples, in which the adsorption capacities decreased by approximately 60?95%. This was presumably because the ground water samples had higher concentrations of competing anions than the other water types.In addition, the iron-based media (GFH, HIOPs, and SMI) had adsorption capacities that were one order of magnitude greater than that of MIEX and AA. HIOPs had the best performance in the Billings water, whereas MIEX and AA were far less effective.
In this study, adsorption measurements were conducted using six adsorbents with different physicochemical properties to characterize arsenic removal from synthetic and natural water samples. The goal was to consistently reduce arsenic concentration to levels below the acceptable level for drinking water (i.e.,10 μg As/L in the US). The kinetic experiment results showed that the adsorption equilibriums for the adsorbents varied depending on the adsorbent type and were achieved within a contact period of 20 min with DI water. Removal of arsenic varied,depending on the different physicochemical properties of the adsorbents. Although experiments were conducted at concentrations one order of magnitude above the most reported occurrence levels, the results are valid as an adsorbent-technology screening tool. The maximum amount of arsenic was observed for MIEX and GFH in pH 5.5 and 7.5 DI water, respectively,whereas HIOPs and SMI were more effective than MIEX and AA for arsenic removal from natural water. This was presumably due to the competition between As(V) and co-ions for the MIEX ion exchange sites. Removal of As(V) was reduced moderately by phosphate for MIEX. However, arsenic removal by AA decreased significantly with an increase in phosphate concentration. The results also indicated that although MIEX outperformed most of the other adsorbents for arsenic removal in anion-free water,MIEX was less effective in natural water samples due to the presence of competing anions. The results indicate that the effects of co- and counter-ions on arsenic removal from natural water should be considered during the development and selection of adsorbents.