Nanofiltration (NF) experiments were conducted to investigate fouling of Al-hydroxide precipitate and the influence of organic compound, inorganic compound, and their combination, i.e., multiple foulants. CaCl2 and MgSO4 were employed as surrogates of inorganic compounds while humic acid was used as surrogate of organic compound. The flux attained from NF experiments was fitted with the mathematical fouling model to evaluate the potential fouling mechanisms. Al-hydroxide fouling with a cake formation mechanism had little effect on the NF membrane fouling regardless of the Al concentration. The NF fouling by Al-hydroxide precipitate was deteriorated in presence of inorganic matter. The effect of Mg was more critical in increasing the fouling than Ca. This is because the Mg ions enhanced the resistances of the cake layer accumulated by the Al-hydroxide precipitate on the membrane surfaces. However, the fouling with Mg was dramatically mitigated by adding humic acid. It is interesting to observe that the removal of the conductivity was enhanced to 61.2% in presence of Mg and humic acid from 30.9% with Al-hydroxide alone. The influence of dissolved matter (i.e., colloids)was more negative than particulate matter on the NF fouling for Al-hydroxide precipitate in presence of inorganic and organic matter.
Nanofiltration (NF) offers an attractive approach to meet multiple water quality objectives, such as removal of organic, inorganic,and microbial contaminants, and thus is a potential alternative to conventional water treatment [1-7]. Also, compared to reverse osmosis (RO) membrane, NF have operational advantages such as lower trans-membrane pressure (TMP), high flux,high retention of multivalent anion salts, an organic molecular weight above 300 Da, relatively low investment, and low operation and maintenance costs [8]. Because of these advantages,applications of NF to treat drinking water have increased in recent years motivated in part by stringent drinking water regulations.The world’s largest NF plant with a capacity of 1,400,000 m3/day is situated in Mis -sur-Oise, close to Paris, France. It provides a population of about 800,000 inhabitants, in the northwest suburbs of Paris with high quality drinking water. The feed water to the NF is pre-treated water from the river Oise [9].
Coagulation as pretreatment to low pressure membrane such as microfiltration (MF) and ultrafiltration (UF) have been suggested to enhance membrane performances by removing particulates, colloidal matter, and particularly, organic compounds,i.e., natural organic matter (NOM) [10-14]. Therefore, coagulation can be used as pretreatment to salt rejecting membranes such as NF and RO which are more vulnerable in colloid and NOM fouling than in the low pressure membranes.
The system used for coagulation pretreatment could be designed with two different trains for membrane filtration. One is coagulation, flocculation, and either sedimentation or filtration to remove aggregates, i.e., floc followed by membrane filtration.Another one is coagulation and then membrane filtration where aggregates are included within feed water of membrane system.Comparing these two trains, it might be expected that the latter method is more appropriate to operate membrane system due to the reduced foot print, complexity, and operational costs.
However, in both pretreatment methods, the aggregate could be one of the major foulants for NF and RO membrane systems.Especially, aluminum (Al) residue in feed water resulting from the poor coagulation treatment can be transformed to Al-hydroxide precipitate as Al residue is influenced by various reaction conditions such as pH and temperature at the membrane surface and/or pores. Waite [15] also mentioned that polymeric Al species with high molecular weight would accumulate at the membrane surface and lead, eventually, to precipitation of an aluminum hydroxide solid.
A hybrid membrane treatment system composed of low pressure membrane and coagulation pretreatment process has become of great interest since it is expected to provide better operation efficiencies [14, 16, 17]. Fouling, however, may become worse due to the floc formed in the coagulation pretreatment process, thus the fouling has been known as the greatest problem in the hybrid membrane process. It has reported that membrane fouling is closely related to the chemical, physical,and morphological properties of the floc which are dependent upon the solute water chemistry, primary particle size in raw water, and coagulation conditions [18-20]. Recently, Listiarini et al. [21] also argued that compared to larger pore membranes, i.e.MF and UF, NF membranes, suffer little or no pore blocking and thus there are possibilities of employing hybrid coagulationnanofiltration technology for membrane treatment.
Some researchers have reported that the use of coagulation pretreatment causes an undesirable effect which is the presence of Al on NF and RO membrane performance [22-24]. Kim et al.[22] observed when employing three different NF pretreatment processes, the ratios of inorganic foulants to the total amounts of foulants are in the sequence of coagulation, sedimentation,and sand filtration water > MF water > Raw water, and in this case the major foulants are iron, silicate, calcium, potassium and aluminum. Park et al. [23] also detected inorganic foulants,i.e., Al and Si, on the fouled NF membrane surfaces and/or pores when different feed waters pretreated by coagulation/sedimentation and sand filtration were used. Additionally, Ohno et al.[24] reported that although the aluminum concentration in the NF feed was not high (30 μg/L), foulants can still accumulate an excess amount of aluminum and silicate, suggesting possible membrane fouling caused by alumino-silicates or aluminum hydroxide.
However, there are still not sufficient studies on the applications of coagulation pretreatment utilizing NF membrane system. Especially, the effectiveness of pretreatment with and without particulate matter, i.e., Al-hydroxide precipitate, was not evaluated. Thus, the objective of this paper is to investigate the Al-hydroxide precipitate fouling on the NF membrane performance such as permeability and rejection. Additionally,fouling of Al-hydroxide precipitate was observed in presence of organic compound, inorganic compound, and their combination.To understand the effect of particulate matter on the NF membrane, MF pretreatment was conducted before any NF experiment.The mathematical fouling model was also adapted to describe the mechanisms of membrane fouling.
2.1. Preparation of Al-hydroxide Precipitates
Polyaluminium chloride coagulant (PACl; 17% Al2O3; Yun Hap Chemical Industry Co., Ltd., Commercial grade, Incheon,Korea) was employed to form Al-hydroxide precipitate, i.e., floc.The PACl coagulant was then diluted to 1.0, 2.0, and 4.0 mM before performing any experiments.
1.0 M NaOH (Showa, Tokyo, Japan) was added to each Al solutions to adjust the pH in the range from 6.0 to 7.0, which is the desirable condition for Al-hydroxide formation since this is suggested by the theoretical calculations using the solubility product constant [25].
Samples were taken without settling, filtered through a 0.45㎛ polypropylene (PP) membrane filter (Whatman, Springfiled Mill, UK), and analyzed for the dissolved Al concentrations by atomic absorption spectrometer (AA6501F; Shimadzu, Kyoto,Japan).
Tap water was used in our study since it provided the solutions with both alkalinity and ionic strength representing natural waters with a low particle concentration. In addition, the tap water was available in a quantity that is sufficient for the continuous operation of filtration experiments together with the disposal of permeate and concentrate. Although the tap waters contain some dissolved chemical species such as Cl?, and SO42-,the interactions between Al and these species were not considered due to their the relatively low anion concentrations compared to the PACl dosage used in this study.
2.2. Addition of Inorganic or Organic Ingredient
CaCl2·2H2O (Showa, Tokyo, Japan) and MgSO4·7H2O (Showa,Tokyo, Japan) was used to investigate the influence of inorganic matter on Al-hydroxide fouling. The concentrations of the chemical solutions were prepared as 5.0 and 3.5 mM for Ca and Mg respectively in the NF experiments.
Humic acid solution used as the organic ingredient was prepared with Aldrich humic acid(Sodium salt, Aldrich, St. Louis,MO, USA) by dissolving 1 g of humic acid in 1 L of deionized water(PURELAB classic; ELGA LabWater, Lane End, UK), and filtering through a 0.45 μm membrane filter (Whatman, Springfiled Mill, UK). The humic acid solution was further diluted using tap water to approximately 10 mg/L as the dissolved organic carbon(DOC) and then applied to NF experiment.
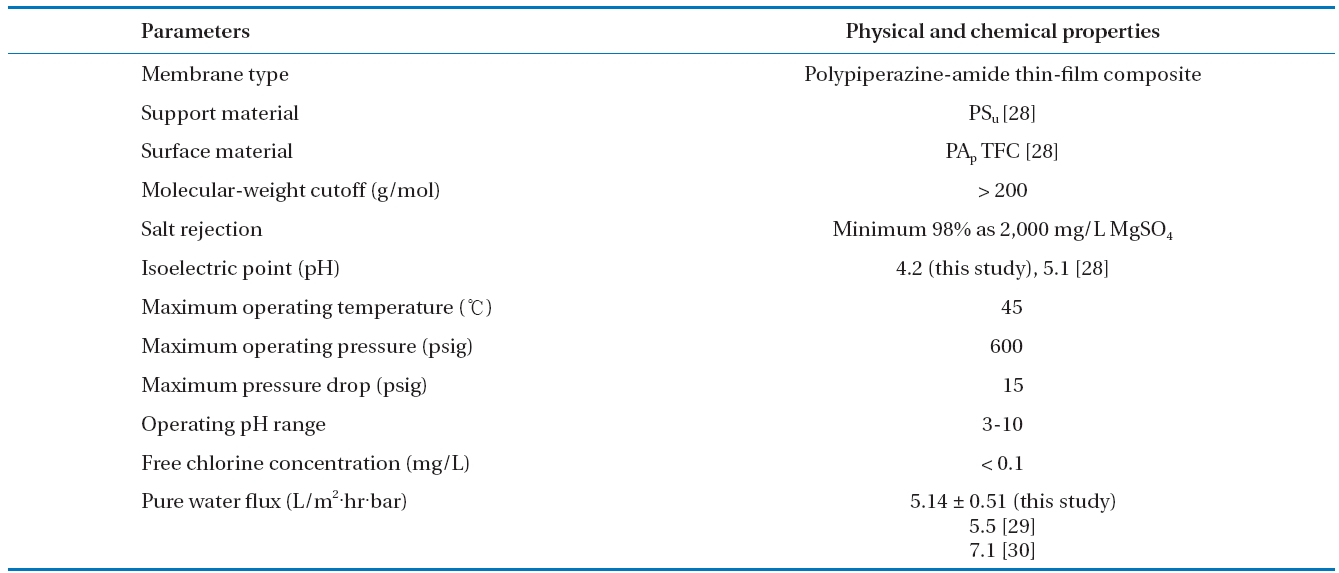
Polypiperazine-amide thin-film composite NF membrane(Dow-Filmtec, Edina, MN, USA), which contains carboxylic and amine functional groups [26, 27], was used throughout the study.The NF membrane has, according to the manufacturer, replaced the discontinued NF 45 series and is primarily designed for process applications that require durable membranes. Also, the NF membrane is designed to remove organic matter with a molecular weight above 200 Da while passing monovalent salts and has a negatively charged surface between pH 4.2 to pH 11 within available operating pH conditions. The characteristics of the NF membrane used were summarized in Table 1.
2.4.1. Cross flow mode system
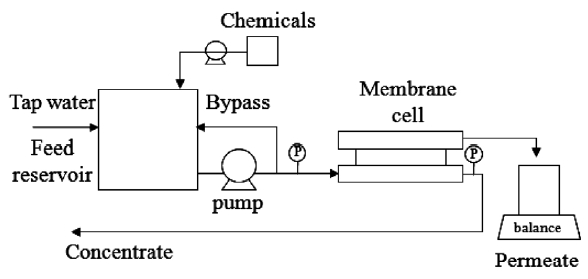
A schematic diagram of the NF apparatus is shown in Fig. 1.A plate-and-frame membrane module (Sepa-CF cell; Osmonics,Minnetonka, MN, USA) was used with a width of 0.095 m,a channel length of 0.146 m and an effective membrane surface area of 0.014 m2. A plastic sieve type feed spacer (Low foulant;Osmonics) stayed inside the cavity on the feeding side of the membrane. The NF membrane filtration system with a 4-L reservoir was operated within a constant pressure (approximately 490 kPa) using the high pressure pump (Wanner engineering,Minneapolis, MN, USA). The tap water with a flow rate of 1 L/min was gathered in the feed reservoir when PACl solution was
[Table 1.] Characteristics of NF membrane used in this study
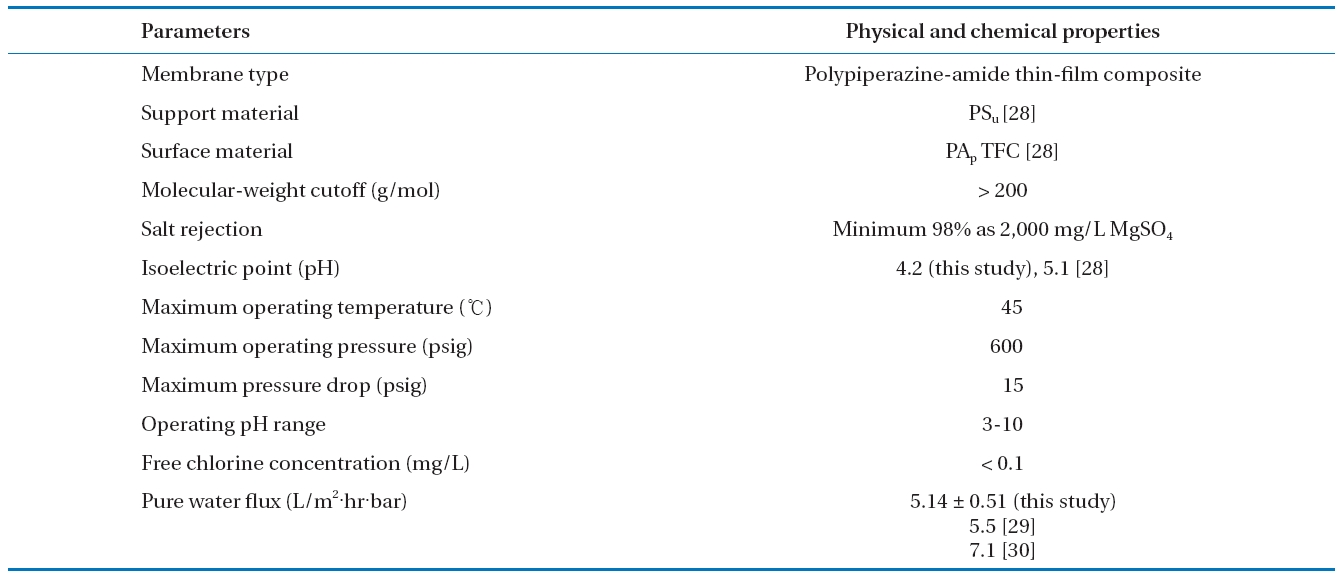
Characteristics of NF membrane used in this study
injected at 3 mL/L simultaneously to adjust the Al concentration of feed water, i.e., 3.5 mM. The feed water pressurized at a flow rate of 0.5 L/min was compressed into the membrane module when the cross flow velocity was 0.9 m/sec on the membrane surface. The filtrate is measured continuously with an electronic balance (AND; Cole-Parmer International, Vernon Hills, IL, USA)to obtain data on the flux decline behaviour. The NF was stabilized overnight with deionized waters. At the start of each run,the membrane permeability was first determined with deionized waters.
2.4.2. Dead-end mode system
The effectiveness of the coagulation pretreatment with and without particulate matter, i.e., Al-hydroxide precipitate, was evaluated by a microfilter (0.45 ㎛, PORPES; Chungsoo Technofil,Guro, Seoul, Korea) that removes particulate matter prior to each NF experiment. Dead-end filtration was employed due to the limited volume of feed water for the NF experiment. Deadend NF filtrations were conducted using batch cells (180-mL capacity;Millipore, Billerica, MA, USA). Each cell was pressurized with a nitrogen gas at 40 ± 0.05 psi using a pressure controller(CZ-68026-58; Cole-Parmer International). An electronic balance(AND; Cole-Parmer International) was used to measure the permeate mass, which was then converted to the permeate volume based on the feed water density. NF membrane used in this system has a surface area of 32.2 cm2. The clean water flux was determined for all fresh membranes using approximately 150 mL of deionized water. The experiment was terminated when the flux of feed water reached the steady state.
2.5. Application of Mathematical Fouling Models
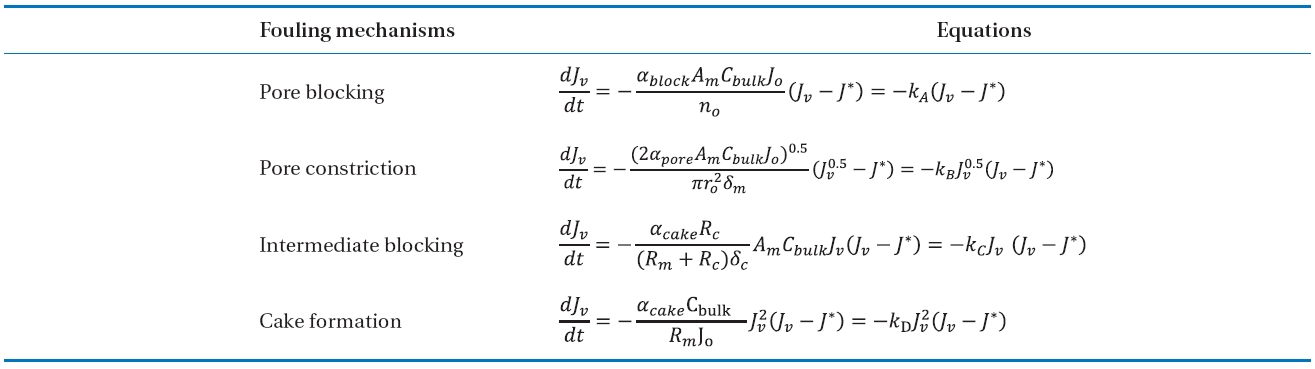
Mathematical fouling models have been developed to explain the permeate flux reduction in the cross-flow membrane operation [31, 32]. Jarusutthirak et al. [32] used the mathematical fouling models to determine the kinetics of membrane fouling and to describe the fouling behaviours during NF as follows:
where
[Table 2.] Summary of fouling mechanisms and equations in the model
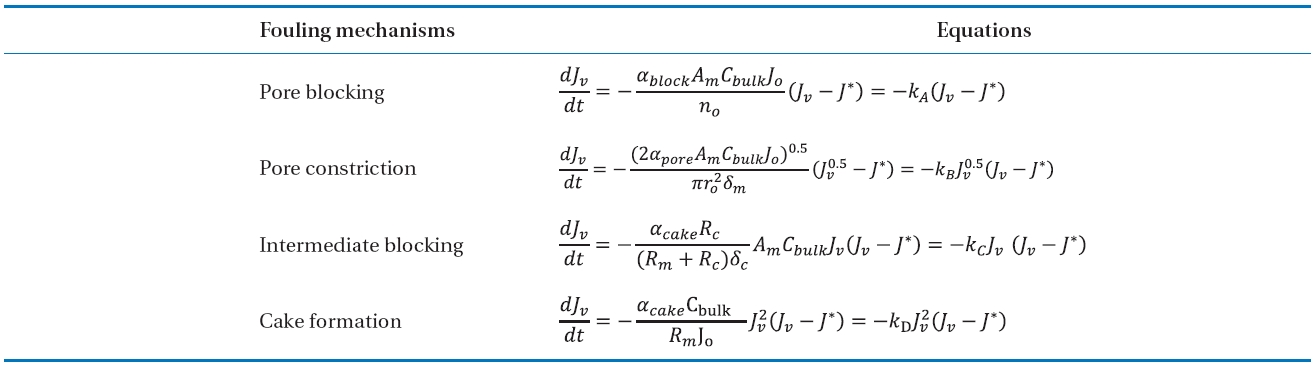
Summary of fouling mechanisms and equations in the model
The divalent cation was analyzed using an atomic absorption spectrometer (AA6501F; Shimadzu). The Al standard solution(Showa Chemical Co., Ltd., Kasugai, Japan) was used to obtain an Al standard curve before each analysis. DOC was measured using a Sievers 5310C Laboratory Total Organic Carbon Analyzer(GE Analytical Instruments Inc., Boulder, CO, USA) equipped with a 900 Autosampler System (Ionics Instruments, Boulder,CO, USA). Potassium hydrogen phthalate (Junsei Chemical Co.,Ltd., Tokyo, Japan) was used as an external standard for DOC analysis. DOC measurements were taken after filtering the samples through the pre-rinsed 0.45 ㎛ membrane syringe filters. A Hach 2100N turbidimeter (Hach, Loveland, CO, USA) was used to measure turbidity and a StablCalⓡ Calibration Set was used for calibration. pH was measured using an Orion Model 410+A pH meter (Thermo Electron Co., Beverly, MA, USA).
3.1. NF of Al-hydroxide Precipitate
3.1.1. Feed water properties
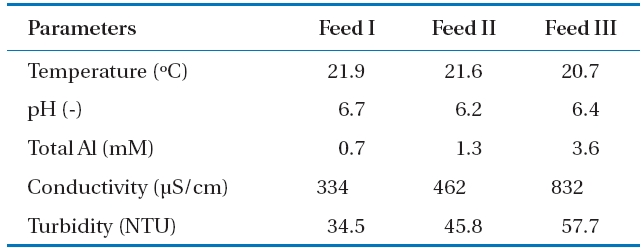
The PACl solutions with three different Al concentrations were adjusted to pH neutral in order to observe the influence of Al-hydroxide precipitate on the NF membrane performance.Al-hydroxide precipitate was formed when the pH of feed waters were adjusted within the range 6.2 to 6.7. Properties of the three feed waters are included in Table 3. Compared to tap water(i.e., background water employed in this experiment), turbidity was not only highly increased approximately from 0.05 to 57.7 NTU but was also related to the increases in the total Al concentrations
[Table 3.] Characteristics of Al-hydroxide solutions with different Alconcentrations
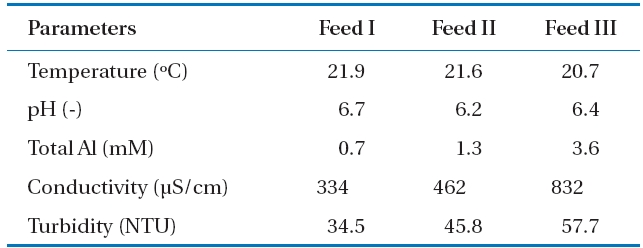
Characteristics of Al-hydroxide solutions with different Alconcentrations
at different feed waters. For instance the turbidities were 34.5, 45.8, and 57.7 NTU that correspond to Feed I, Feed II,and Feed III, respectively. A considerable quantity of particulate matter was Al-hydroxide precipitate such as Al(OH)3. The conductivity of the solutions was also increased due to the relatively large dosage of PACl used for Al concentration adjustment and NaOH for pH controlling from Feed I to Feed III.
3.1.2. Evaluation of NF performance
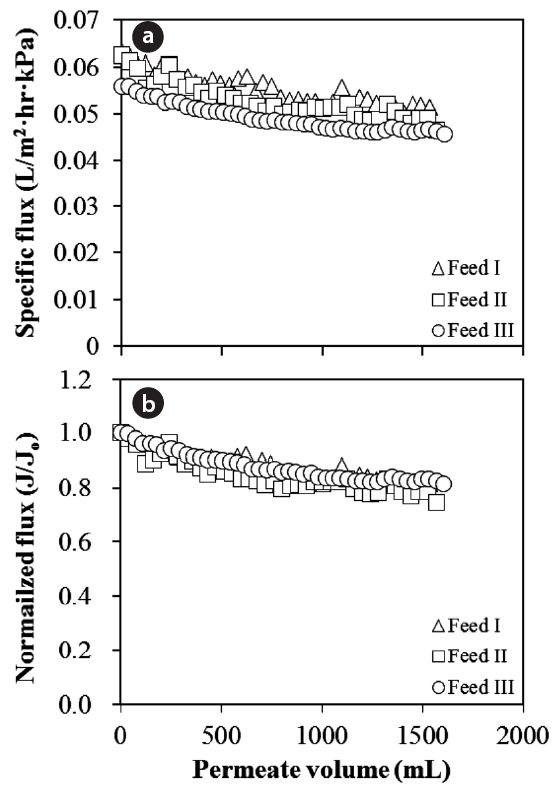
In general, the flux decline and rejection rate are tools used to evaluate the membrane performance. As one of the parameters,the behavior of flux decline was applied to investigate the impact of Al-hydroxide precipitate with different Al concentrations on NF membrane fouling. The permeate flux was defined as the permeate volume per unit membrane area during a certain filtration time. The specific flux is result when the permeate flux was divided by the trans-membrane pressure. When the permeate and/or specific flux was divided by the initial flux, a normalized flux could be obtained. The normalized flux is useful to compare the flux decline behaviors of solutions with different initial flux values. For each set of experiments, the flux decline is presented in two different forms, namely the specific flux (Fig.2a) and the corresponding normalized flux (Fig.2 b).
In fact, a significant flux decline under the impact of Alhydroxide was expected possibly due to the accumulation of particulate matter with high turbidity on the membrane surface.However, during the NF experiments, little flux decline was observed. Moreover, the Al concentrations showed no considerable effect on membrane fouling even at high turbid concentrations,i.e., from 34.5 to 57.7. This indicates that both the Al and particulate matter concentrations were not important factors to NF membrane fouling. The fouling phenomenon therefore suggested that positively charged polymeric Al in the PACl solution would be neutralized by OH resulting in Al-hydroxide precipitate with no electrical surface charge. As a result, Al-hydroxide fouling with little flux decline was attributed to merely the accumulation of the precipitate with the abundant turbid matter on the membrane surfaces. Furthermore, it was reported that the amorphous Al-hydroxide can be formed with an OH/Al ratio of 3.0 [33]. Such amorphous precipitates might have a positive effect on the membrane fouling, possibly related to the formation of a porous and low resistant fouling layer without inner and/or external pore fouling at the membrane surface, which leads to the mitigation of severe membrane fouling.
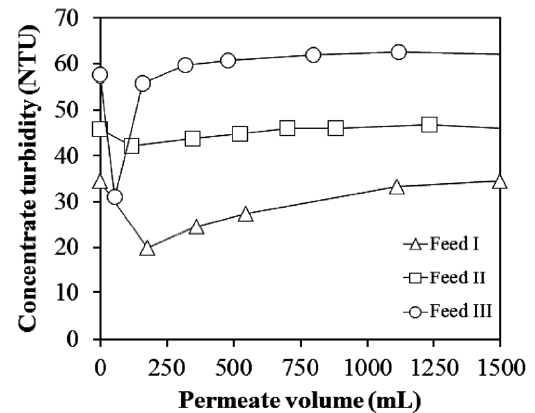
To identify the accumulation of Al-hydroxide precipitate, the turbidity within the concentrate was measured during the NF
experiment with different feed waters. As shown in Fig. 3,the turbidity was lowered at the initial period of membrane filtration,compared to the feed water. This result illustrated that the Al-hydroxide precipitate was accumulated on the membrane surface at the start of the filtration and also reveals that the cross flow velocity of 0.9 m/sec applied in this NF experiment was not a crucial factor to control the deposition of the precipitate on the membrane. The turbidity was then gradually increased and reached a constant value close to the feed water turbidity. After which, the accumulation of Al-hydroxide on the membrane surface was thought be limited.
Consequently, coagulation pretreatment without settling could be employed in a NF membrane system since the impact of the coagulated flocs was not significant on the NF membrane fouling. Listiarini et al. [21], also argued that compared to the larger pore membranes, i.e., MF and UF, NF membranes experience little or no pore blocking and thus there are possibilities of employing hybrid coagulation-nanofiltration technology in the removal of organic matter.
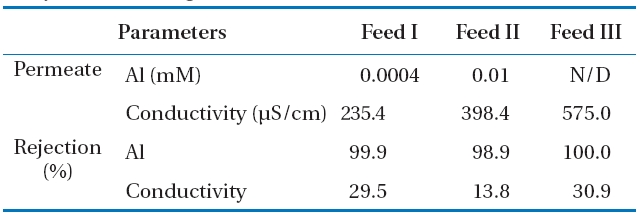
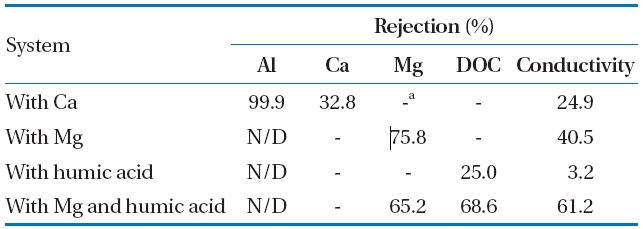
Other parameters used to evaluate the membrane performance are the rejection characteristic of Al and the conductivity during Al-hydroxide precipitate filtration with NF membrane.The permeate quality and rejection is presented in Table 4.
Most of the Al was rejected on the NF membrane regardless of the Al concentration of feed waters, since the Al rejection was 99.95% at 0.7 mM Al (Feed I), 98.89% at 1.3 mM Al (Feed II), and 100% at 3.6 mM Al (Feed III). Unlike the Al rejection, conductivity rejection was significantly lower at 29.5%, 13.8%, and 30.9%for Feed I, feed II, and Feed III, respectively.
As described in the literature, the surface charge of NF membrane plays a critical role in the rejection of charged ion [8, 34,35]. It is briefly summarized as the electrostatic repulsion, i.e.,Donnan exclusion, between the charged ion and membrane surface. Therefore, the conductivity rejection might be rationalized as the cake layer accumulated by Al-hydroxide precipitate which shielded the negative charge of membrane at the neutral pH condition and consequently caused weak repulsion forces between the membrane and the charged ion.
Gabelich et al. [36] also reported that the salt rejection was decreased in RO membrane systems with coagulation pretreatment using PACl coagulant, in which the residual Al concentration was 50 μg/L at ambient pH conditions (pH 7.8-7.9). Additionally,Schrader et al. [37] suggested that the decrease in salt rejection as a result of the Al addition to the feed water at pH 8.0 led to a decreased membrane surface potential, i.e., the loss of electrostatic repulsion.
3.2. Influence of Inorganic and Organic Matter
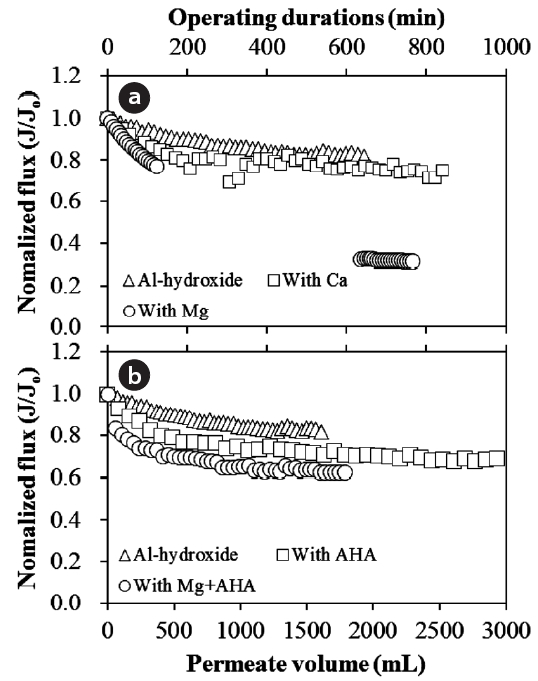
To compare the effect of different compounds on Al-hydroxide precipitate fouling, the normalized flux decline for the fouling in presence of Ca, Mg, humic acid, and their combination,i.e., Mg and humic acid, were presented in Fig. 4 where for all three cases the flux decline rates were increased.
Overall the flux decline rate was only slightly increased in presence of humic acid. It is postulated that the increased resistances
[Table 4.] Characteristics of rejection and permeate water quality forAl-hydroxide fouling
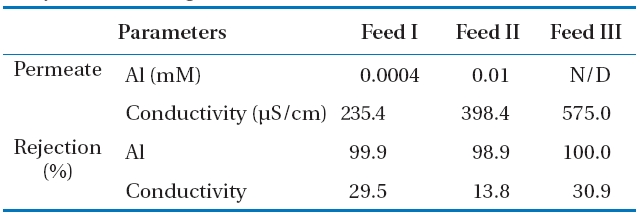
Characteristics of rejection and permeate water quality forAl-hydroxide fouling
of cake layer formed by Al-hydroxide in presence of humic acid results in a more decreased flux decline rate compared to the Al-hydroxide precipitate fouling without humic acid. It is also noted that the high humic water flocs (i.e., Al-hydroxide precipitate) were more compressible under external pressures,which can substantially increase the cake resistance [38].
The impact of the divalent cation on Al-hydroxide precipitate fouling was more substantial in presence of Mg than Ca.This shows that the impact of Mg on the increase of the cake layer resistance was more significant than Ca. Additionally, the flux decline with Al-hydroxide precipitate and humic acid was further reduced by introducing Mg into the solution. This suggests that the resistance of the combined cake layer formed by Al-hydroxide and humic acid could be further enhanced in presence of Mg. In the previous study on organic fouling of loose NF membranes [39], Mg have been shown to increase the organic fouling rate attributed to the electrostatic interaction with NOM. However the effect of which was not as significant when compared to Ca due to an intermolecular bridging mechanism.On comparison, the intermolecular bridging mechanism of Ca found in organic fouling was not significant in presence of colloidal matter [40].
At the Al-hydroxide precipitate fouling on the NF membrane,Mg dramatically reduced the flux decline of the initial water specific flux by 68.4% compared to 37.6% of initial water specific flux with Mg and humic acid after a permeate volume of approximately 3,500 mL. Unlike the negative effect of Mg on the Al-hydroxide precipitate fouling, humic acid played a critical role in the release of the suggested resistance of the cake layer formation along with Mg despite the membrane fouling for Alhydroxide alone could be further deteriorated in presence of Mg and humic acid, possibly due to different cake properties.
The rejection feature of the NF membrane was observed for Al-hydroxide precipitate fouling in presence of inorganic compound,organic compound, and their combination. As shown in Table 5, the Al rejection was more than 99.9%, which demonstrated the high Al selectivity of the membrane. However, as discussed above, the charge of the membrane surface may have been neutralized, due to the accumulation of the Al-hydroxide precipitate, resulting in the increased ion transport through the NF membrane without electrostatic repulsion.
Moreover, the rejection of conductivity was 3.2 to 61.2% at each experiment system. Of which the humic acid was a significant factor decreasing the conductivity rejection by 3.2%.It should be noted that humic acid in feed water was primarily controlled to prevent the deteriorating removal of conductivity for Al-hydroxide precipitate fouling in coagulation followed by NF membrane system. In addition, even though a low DOC concentration due to the removal of DOC by Al-hydroxide precipitate is introduced to the membrane, relatively low DOC rejection of 25.0% was observed in presence of humic acid. However,when both Mg and humic acid were added to the solution of Al-hydroxide precipitate, the rejection of DOC and conductivity was increased by 68.6 and 61.2%, respectively.
3.3. Influence of MF Pretreatment
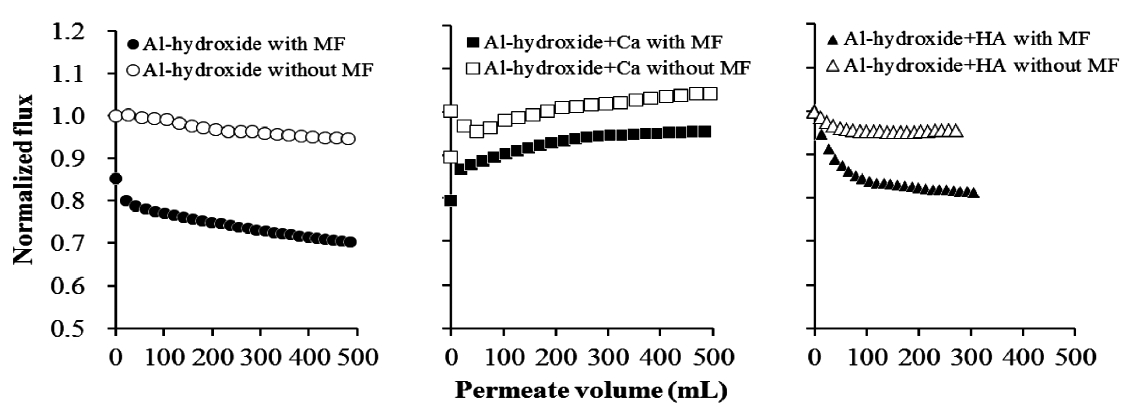
Effect of the MF membrane pretreatment on NF membrane fouling by Al-hydroxide precipitate was investigated. Interestingly,Fig. 5 shows that the MF membrane pretreatment did not mitigate the Al-hydroxide precipitate fouling but increased the rate of flux decline. The negative effect of MF membrane pretreatment was most severe for feed water with Al-hydroxide precipitate alone. This result suggests that the dissolved matter with size of 0.45 ㎛ below were major foulants such as colloids for Alhydroxide precipitate fouling on NF membrane with MF membrane pretreatment. In fact, a significant impact of Al-hydroxide precipitate on NF membrane might be expected possibly due to the formation of fine particles.
Bertsh et al. [41] suggested that fine particulate matter such as colloidal solids could be formed at the initial coagulation reactions and also Pernitsky and Edzwald [25] mentioned that microcrystal particles with a size range of 1 nm to 200 nm could be formed when PACl was used for coagulation treatment. Inversely,it is worth to note that the particulate matter may also contribute to the mitigation of the dissolved matter fouling even though the mechanism of fouling reduction proposed in this paper could not rationalize this.
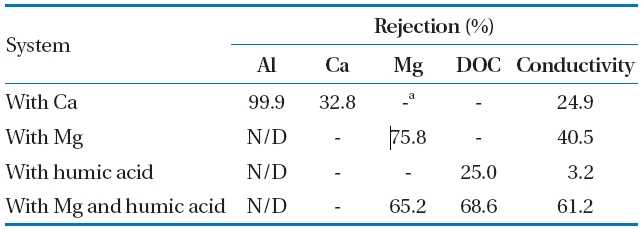
Rejection characteristic for Al-hydroxide precipitate foulingin presence of organic compound inorganic compound and theircombination
Variable fouling mechanisms and critical flux calculated from mathematical model for combined Al species fouling
Al-hydroxide fouling both with and without MF membrane pretreatment was reduced in the presence of Ca while a significant flux decline rate at initial stage of NF operation was gradually increased to the initial membrane flux during the NF membrane experiment. Different from the result at the cross flow system, the operating condition, i.e., cross flow and/or deadend mode, of the NF membrane system could affect the role of Ca on Al-hydroxide fouling, postulated to the different fouling mechanism.
In summary, MF membrane pretreatment revealed negative effects on NF membrane fouling of Al-hydroxide precipitate in presence and absence of Ca and humic acid. The flux decline rate of the feed waters with the MF membrane pretreatment was increased more than the one without the MF pretreatment.The results suggest that the “dissolved” species such as colloids participated in the membrane fouling in a greater extent than particulate matter for Al-hydroxide precipitate. Most of the NF membrane experiments in this study did not employ pretreatment such as settling and/or filtration of feed waters. The installation of a filter such as cartridge filter, microfiltration, or ultrafiltration,is recommended to protect the high pressure pumps for NF in real plants. However, results presented here suggest that the installation did not reduce NF fouling.
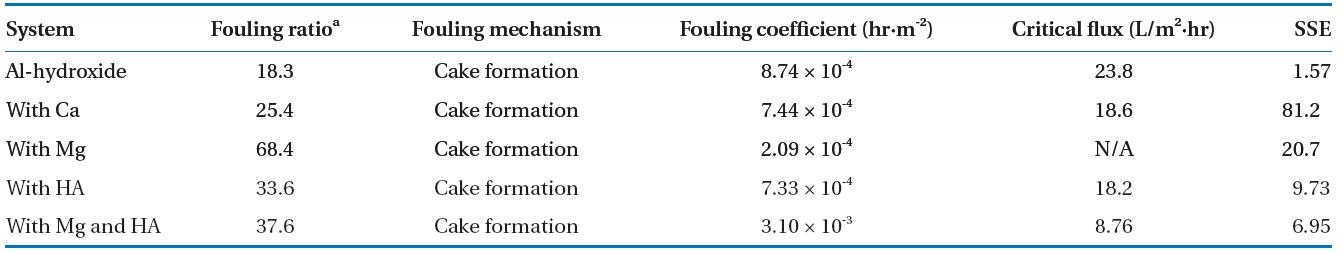
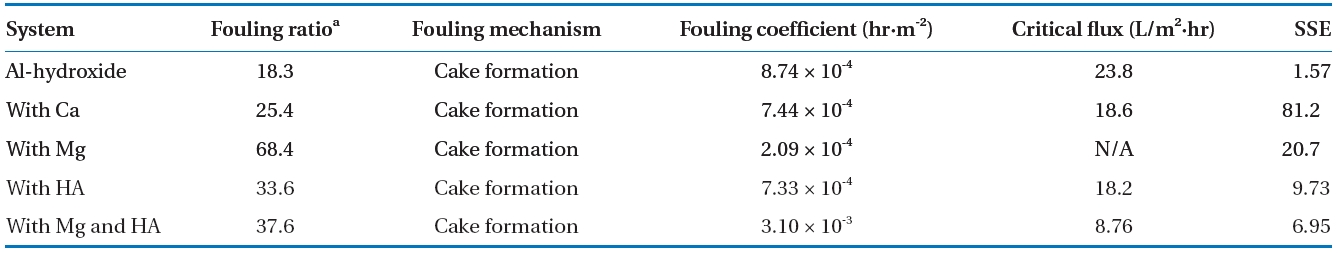
The models proposed by Jarusutthirak et al. [32] were used to estimate the fouling mechanisms associated with Al-hydroxide precipitate. Table 6 presents the calculated parameters from the fouling model with fouling ratio presented above results. These results were based on minimizing the sum of squared errors(SSE) between the experimental data and the estimated data from each of the fouling models (Σ[Jv(model)-Jv(measured)]2).The model with the smallest SSE could be considered as a more accurate fit, and thus a more feasible fouling mechanism for a given solution.
Of which, the cake formation mechanism was the best fit.This therefore indicates that the Al-hydroxide precipitate larger than the pore size of the NF membrane was apparently accumulated on the membrane surfaces resulting in formation of cake layer. Also, this results support the formation of a porous and low resistant fouling layer without inner and/or external pore fouling at the membrane surface, which avoids severe membrane fouling. However, when the same fouling mechanism was adapted for different feed waters, the behavior of membrane fouling was varied in the range of 18.3 to 68.4% as a fouling ratio.Such variations might be attributed to certain properties of the cake layer on the membrane surfaces such as specific cake resistance and compressibility [1].
The critical flux,
This indicates that the Al-hydroxide precipitate fouling was most severely occurred in presence of Mg and humic acid simultaneously,but the significant flux decline might be avoided when NF membrane system was operated approximately 8.8 LMH below initial flux.
In addition, the fouling mechanisms of Al-hydroxide with and without Mg and humic acid are suggested to be the same as cake formation but with more flux decline rate when both Mg and humic acid are present. The KD (h·m-2) which indicates the rate constant or fouling coefficient for cake formation was calculated to be 8.74 × 10-4 and 3.10 × 10-3 for Al-hydroxide alone and in presence Mg and humic acid, respectively. This result agrees well with the increased cake resistance of Al-hydroxide resulting from the presence of Mg and humic acid on the membrane surfaces.
The Al-hydroxide precipitate fouling on the NF membrane performance was investigated in presence of organic compound,inorganic compound, and their combination. Also, the influence of MF on NF membrane system with coagulation pretreatment was evaluated. In particular, remarkable conclusions that can be drawn from this research are as follows:
1) Al-hydroxide precipitate had little effect on the NF membrane fouling with a low flux decline rate regardless of the particulates concentration, i.e., turbidity resulting from the increase in Al concentrations. Al-hydroxide precipitate accumulated on the membrane did form a cake layer which was one of the major factors avoiding severe flux decline.
2) The NF rejection of conductivity was significantly deteriorated by Al-hydroxide precipitate fouling. The cake layer led to weak repulsion forces between the membrane and the charged ionic contents.
3) Mg dramatically increased the Al-hydroxide precipitate fouling for NF. However, humic acid played a critical part in the resistance reduction of the cake layer formed by Al-hydroxide along with Mg. It is most likely that the fouling with inorganic compounds replies on the presence and absence of organic matter possibly due to different cake properties.
4) The Al-hydroxide precipitate may contribute heavily to the mitigation of dissolved matter fouling and therefore coagulation without settling and/or prefiltration could be used as a pretreatment method for the NF membrane systems.