Color changes in fish are generally divided into two types: temporary reversible changes and permanent irreversible changes, based on the time period and morphological characteristics involved.With reversible changes, skin colors can change depending on the situation and then return to their original color, whereas with irreversible changes,once the skin color changes, it never returns to the original. Centripetal and centrifugal movements of pigments in stellate chromatophores are believed to be the cause the former situation when the pigment cells complete their differentiation. This process is driven by many factors, including background pattern(Ramachandran et al., 1996), stress (Doolan et al.,2008), luminosity (Han et al., 2005), and nutrition(Kalinowski et al., 2005). The latter occurs when the number of chromatophores changes due to the differentiation of pigment cells, which is thought to be caused by inherited gene characteristics (Shikano,2005; Jeong and Jeon, 2008; Kang and Lee, 2010),the existence of light (Iwata and Kikuchi, 1998),habitat conditions of habitats (Ottesen and Strand,1996; Iwata and Kikuchi, 1998), the colors in surroundings (Amiya et al., 2005; Yamanome et al., 2005,2007), and the stocking density (Takahashi, 1994).
In teleostes, high rearing densities are known to induce stress-induced temporary color changes(Doolan et al., 2008), bone deformation (Boglione et al., 2009), and smoltification (Hosfeld et al., 2009)and to affect growth (Bolasina et al., 2006; Merino et al., 2007), survival (Tagawa et al., 2004), sex ratio(Saillant et al., 2003), sexual maturation and spawning (Claudia et al., 2004), hatching (Peck and Holste, 2006), and cannibalism (Baras et al., 2003).However, whether density affects the differentiation and proliferation of pigment cells during the development of flatfish such as the olive flounders remains unknown.
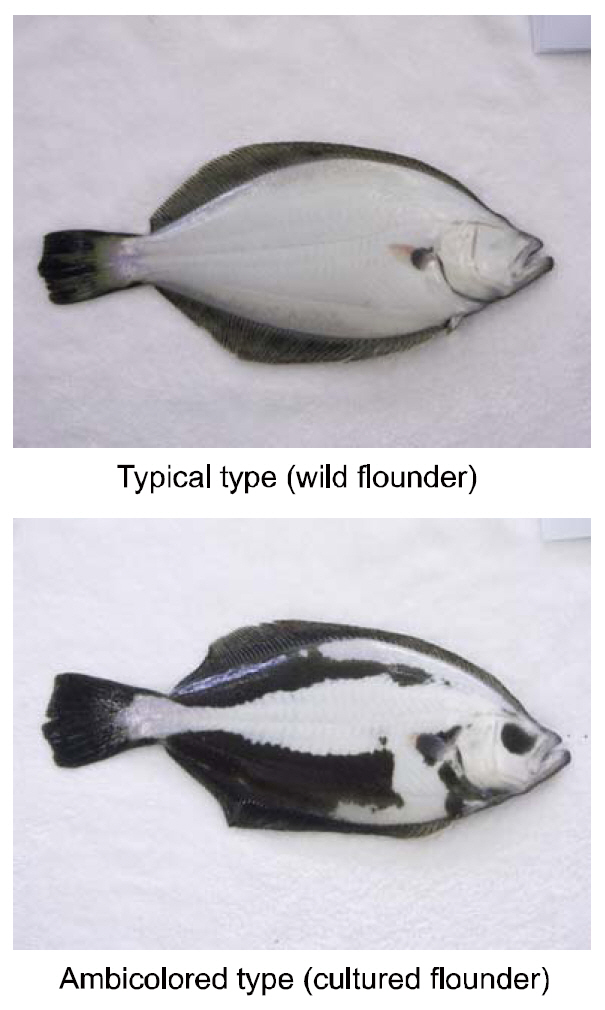
The hypermelanosis of the blind side(ambicoloration) in olive flounders
[Fig. 1.] Photographs of the blind side of wild and cultured olive flounders Paralichthys olivaceus.
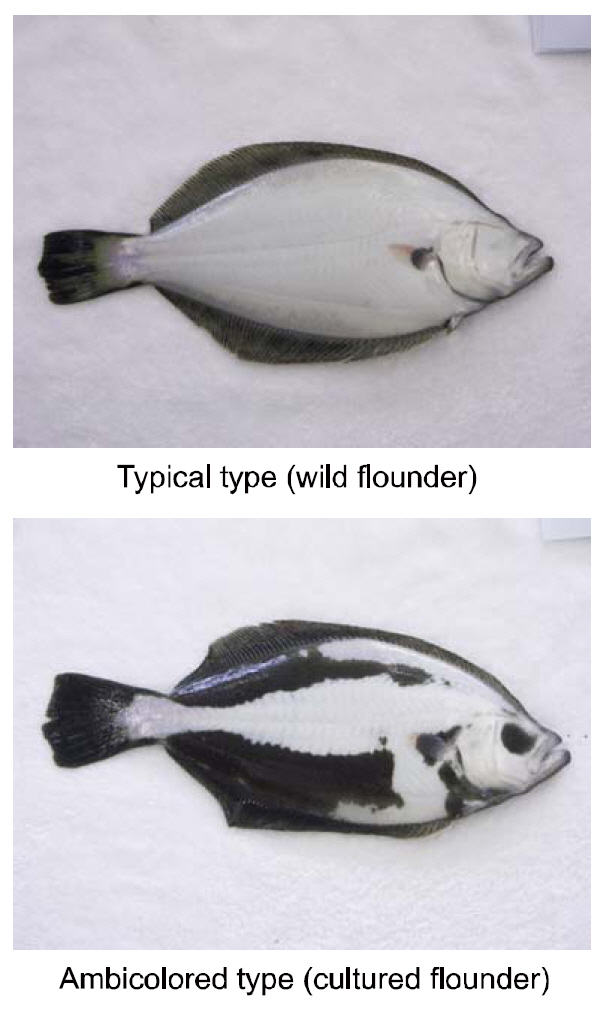
Considering that wild adult and juvenile flounders are generally solitary, the stress of high rearing densities may induce the staining on the blind sides of artificially cultured fish. Bolasina et al., (2006) reported that fish do excessive stress when reared at high density in artificial facilities, and Takahashi(1994) identified a link between staining on the blind side and rearing density. However, several other studies have proposed factors other than rearing density as a cause for the malpigmentation (Ottesen and Strand, 1996; Iwata and Kikuchi, 1998; Haga et al.,2004; Amiya et al., 2005; Yamanome et al., 2005, 2007).Thus, whether density is the main driving factor is not clear. One must therefore determine whether rearing density is the main or secondary factor in ambicoloration. Here, we studied the relationship between rearing density as a stress-inducing agent and the blind-side hypermelanosis of olive flounders.
The experimental 75-day-old fish were reared in grey 20 t concrete tanks at the density of 200 fish/m2/t using flowing natural seawater (mean temperature 18.3±2.4°C, salinity 30.7±0.4‰, and dissolved oxygen [DO] 7.1±0.5 mg/L) with artificial lighting that simulated a natural photoperiod. To assess the effects of rearing density on the ambicoloration of olive flounders, 1,200 size-matched (total length [TL] 6.6±0.1 cm, body weight [BW] 2.55±0.04 g) fish not entirely stained on the blind side were selected and then divided in duplicate into two groups: at 150 animals/m2 tank (commercial production density:control) and 450 animals/m2 tank, respectively.
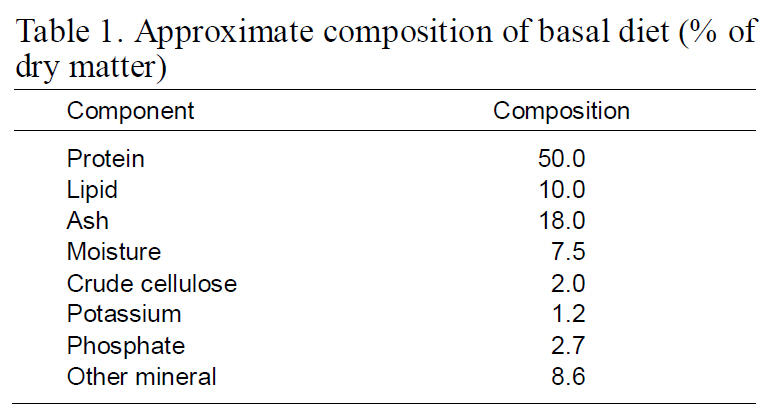
The fish were transferred into a 1,000 L dark-green FRP tank (base surface area of 1-m2/wall surface area of 1-m2) supplied with filtered seawater (one to fivefold water exchange rate per day depending on biomass) and reared with gentle aeration and artificial lighting simulating a natural photoperiod at a temperature of 19.7±3.2°C, salinity of 30.9±0.3‰, pH of 7.7±0.3 and DO of 7.3±0.8 mg/L. The flow rates were identical in all tanks. Tank bottom were cleaned once a day by siphoning. With the exception of the sampling day, fry were fed twice daily to satiation with a commercial pellet diet (SCF, Incheon, Korea) (Table 1). The high-density rearing treatment was conducted for 90 days.
We recorded the mass of feed consumed by each group during the experiment, and determined the daily
[Table 1.] Approximate composition of basal diet (%of dry matter
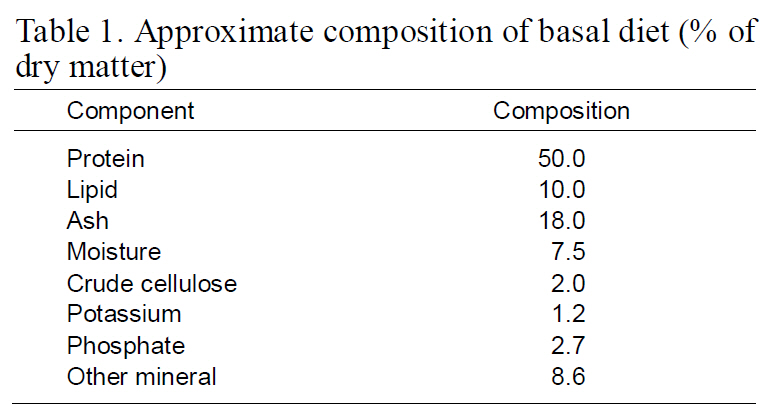
Approximate composition of basal diet (%of dry matter
feed intake (DFI=total mass of food/ [days×fish], mg/fish/day) and the feed conversion efficiency (FE=body mass gain/mass of diet fed×100, %). Daily mortality records were kept to calculate survival in each group. We measured the total length (cm) and body mass (g) of each animal on the first and last day.The fish were not fed for 24 h prior to sampling, and they were anesthetized in 100 mg/L MS-222 buffered with 100 mg/L sodium bicarbonate. The daily growth rates in length (DGRL, %=[
>
Ratios of ambicolored fish and staining on the blind side
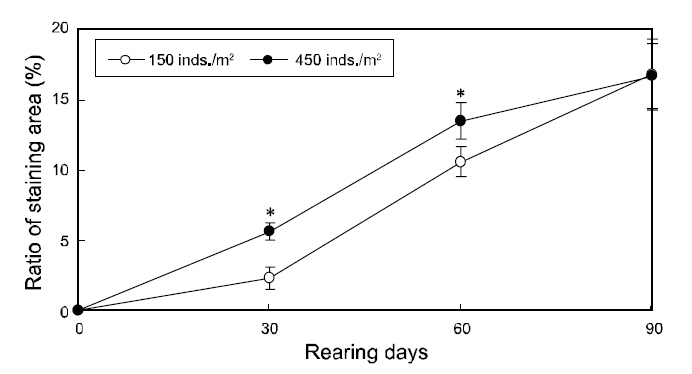
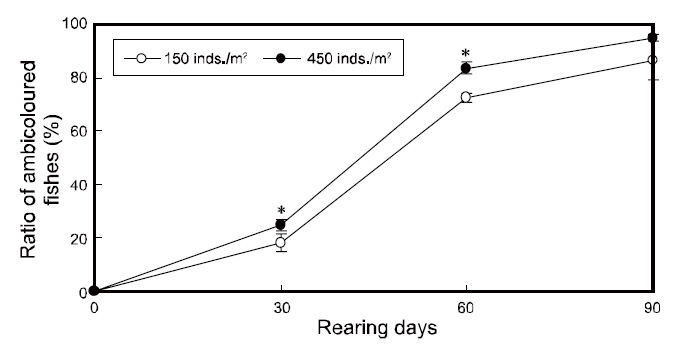
To investigate the effects of rearing density on the abnormal pigmentation of the blind side of olive flounders, 30 flounders were collected in duplicate from each tank using a net (n=60 fish per group), at an interval of 30-90 days. For sampling, the fish were euthanized by 2-phenoxyethanol (1/1,000 dilution,0.3-0.4-mg/L), as an general anesthesia, and then were rinsed with distilled water to remove salt. Next, these samples were kept in 10% neutral formalin bottles. The blind sides of sampled flounders were photographed using a digital camera (DFC 320; Leica, Wetzlar, Germany) with a built-in stereomicroscope at the end of the experiment. Then, the ratio of the staining area (%=staining area of the blind side/total area of the blind side×100) between each group was analyzed on the final day using a micro-imaging analysis system (Qwin; Leica, Wetzlar, Germany) with the previously taken image. Individuals exhibiting staining of greater than 1% of the blind side area were considered to be ambicolored. The total number of ambicolored individuals was determined for each group and the ratio of ambicolored fish (%=ambicolored fish/total fish×100) was calculated at 30 day intervals.
Statistical analyses were conducted to detect significant differences in the mean values between the two groups. DFI, FE, DGR, and survival rate were compared using the mean of two subsamples of 30 fish as replicates (n=2), and TL, BW, CF, and the ratios of staining area and ambicolored fish were compared using the mean of 60 pooled sample individuals as replicates (n=60). Using the statistics program SPSS version 7.0 (SPSS Inc., Chicago, IL, USA), a Mann-Whitney U-test in DFI, FE, DGR and survival, and
>
DFI, FE, growth, and survival
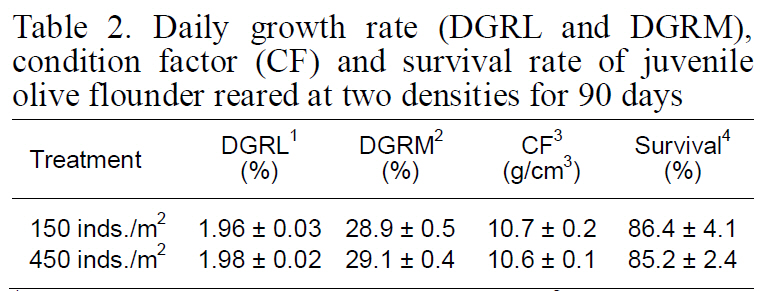
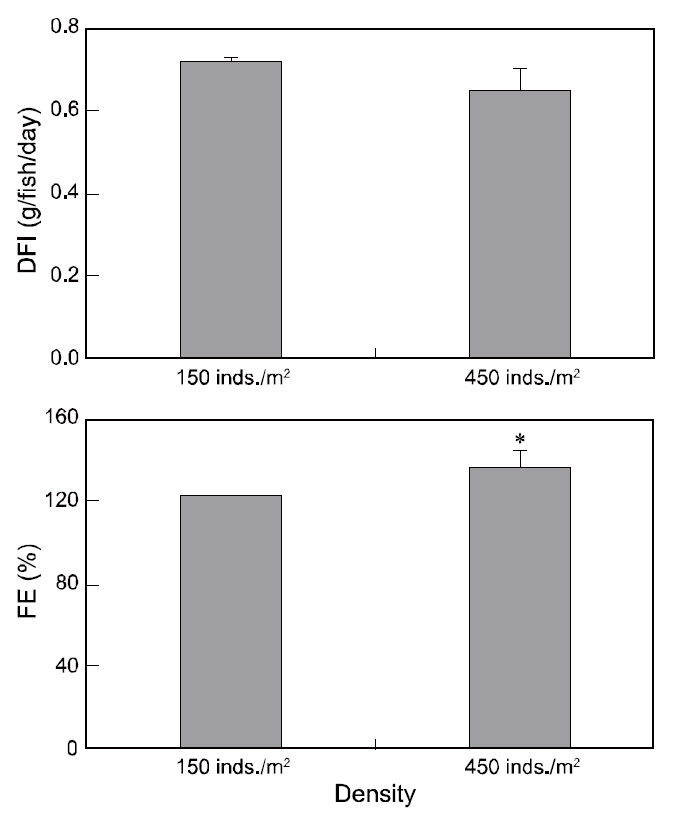
Although DFI was not significantly different between the control and high-density groups, the DFI in the low-density setting (control) was a slightly higher than that in the high-density environment. Fish in the high-density group exhibited higher FE than those at low-density (Fig. 2). However, no significant difference was observed in the growth rate, CF, or survival of fry between the two experimental groups(Table 2).
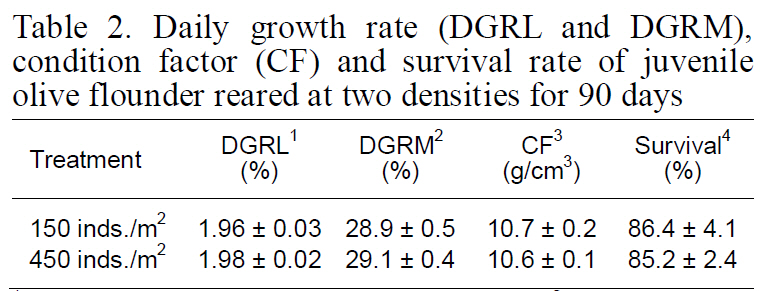
Daily growth rate (DGRL and DGRM)condition factor (CF) and survival rate of juvenile olive flounder reared at two densities for 90 days
>
Pigmentation on the blind side
The changes in the ratios of staining area in the two groups throughout the testing periods are shown in Fig. 3. The ratios of staining area in both groups increased from zero to 90 days of growth and was significantly higher under high-density conditions from 30 to 60 days; however, the ratios were similar in the two groups by the end of the experiment period(Fig. 3).
In agreement with previous reports, the ratio of ambicolored fish was significantly higher in the high
density group from 30 to 60 days of growth,indicating that the pigmentation on the blind side occurred more quickly under high-density conditions(
Our morphological observations showed that the blind-side malpigmentation as a result of differentiation and development of pigment cells (presumably melanophores, xanthophores and iridophores), which differentiate in the same way as on the ocular side,and as has been reported by Kang et al. (2010), is an irreversible change. The cause and mechanism of the hypermelanosis remain unclear, but are assumed to be a consequence of interactions between epigenetic (Kang and Lee, 2010) and environmental factors(Takahashi, 1994; Ottesen and Strand, 1996; Yamanome et al., 2005). Additionally, the factor that has the strongest impact on the irreversible pigmentation,and the mechanism involved in the development of this phenomenon that is so rarely seen in wild olive flounders remain unknown.Specifically, to determine the causes of abnormal pigmentation and to develop a mitigating technology,a physiological comparison of normal and abnormally staining fish is required to pinpoint stresscausing agents in farming. Therefore, based on Takahashi (1994), we studied the effects of rearing density as an environmental stress factor on blindside hypermelanosis in olive flounders.
Our results demonstrated that stock density does not ultimately influence the blind-side hypermelanosis of olive flounder. The staining area and ambicolored fish ratios in both groups increased significantly from 0% to more than 15% and 80%,respectively, by the final day (90 days). However,given the present results, the rearing density can
account for a portion of the hypermelanosis, and can induce rapid progression of the symptom before 60 days of rearing. Although both groups showed similar feed intake, growth, and survival performances and the staining area ratio was equal between the groups by the end of the experiment, a high-rearing density does appear to increase the ratios of staining area and ambicolored fish from 0 to 60 days. These results indicate that high-density farming accelerates the bilateral body pigmentation of olive flounders, which is partially consistent with the results reported by Takahashi (1994), which suggested that abnormal pigmentation increases with stocking density. The apparent influence of density on the speed of malpigmentation on the blind side in flatfish may be due to a stress stimulus induced by increased crowding. Recently, Kim et al. (2008) found that ambicolored flounders exhibit higher stress levels than fish with normal coloring suggesting that abnormal pigmentation may be related to stress (Bolasina et al., 2006). Therefore, we propose that high-density farming conditions increase the stress levels of flounders, which in turn causes the growth of pigment cells on the blind side. This may represent a recurrence of cells that once served defensive camouflage purposes, which have since degenerated with evolution. However, note that the accelerating effect under high-density conditions was lost after 60 days. We suggest that this occurred because the total biomass per unit area in the low-density tanks increased as the fish grew, perhaps raising the density to a threshold at which these flounders experienced high-density stress levels. The effect of increased stress with time might also account for the delayed staining in the low-density group. This may have triggered the growth of the once-atrophied pigment cells on the blind sides of the fish, which it had reached the levels of the high-density group by day 90.
Here, we found that increased rearing density does not affect overall the blind-side staining, but accelerates the initial hypermelanosis of olive flounders. Our results suggest that crowding plays a role in the hypermelanosis of olive flounders, but that it is not the only environmental stress factor behind this phenomenon. Therefore, why ambicoloration only occurs under artificial rearing remain a mystery.Ambicoloration can be explained in two ways: as a morphological mutation by change of genomic sequence, or as a type of atavism in which ancestral traits reappear by silence phenotype and gene expression in genetics. We are in support of the latter opinion, proposed by Ivankova and Ivankov (2006) and Ivankov et al. (2008), because both partial and complete ambicoloration are clearly related to the evolution of flounders. Flounders (or Pleuronectiformes) are reported to have originated from ancestors close to either Zeiformes or Percopsiformes related to Percoidei (Ivankova and Ivankov, 2006; Ivankov et al., 2008). Recently, Friedman (2008) suggested flatfish evolved from symmetric fish, which lends credibility to the claim that flounders evolved from Zeiformes or Percopsiformes. We propose that pigment cells on the blind side, which had vanished during evolution, reappeared functionally by silence phenotype and gene expression under inadequate living conditions. Future studies of the possible relationships between epigenetic change and blindside pigmentation as an evolutionarily regressive trait are warranted.
In conclusion, although rearing density is not the main cause of the hypermelanosis on the blind side of olive flounders, an increased rearing density can accelerate the blind-side malpigmentation in olive flounders as was demonstrated by the number of ambicolored individuals and the area of the blind side.This is an important factor in this phenomenon that should not be overlooked.