Conducting polymer-coated multiwalled carbon nanotubes (MWCNTs) were prepared by template polymerization in order to enhance their gas sensitivity. This investigation of the conducting polymer phases that formed on the surface of the MWCNTs is based on field-emission scanning electron microscopy images. The thermal stability of the conducting polymer-coated MWCNTs was significantly improved by the high thermal stability of MWCNTs. The synergistic effects of the conducting polymer-coated MWCNTs improve the gas-sensing properties. MWCNTs coated with polyaniline uniformly show outstanding improvement in gas sensitivity to NH3 due to the synergistic combination of efficient adsorption of NH3 gas and variation in the conduction of electrons.
The importance of environmental gas monitoring is well understood, and many studies have focused on the development of suitable gas-sensing materials [1]. The toxic gases generated in many places such as plants and laboratories cause adverse effects on the human body and generate acidic rain, which devastates the environment. Consequently, a great deal of research is being conducted on gas sensors [2-4].
Most of the gas sensors available on the market are inorganic metal oxide semiconductor-based sensors which can be operated at high temperature to ensure the sensitivity and selectivity of the sensors [5-7]. However, new sensing materials are expected to have a high gas-sensing ability at room temperature [8]. Conducting polymers, for example, can be used as a sensitive layer of a gas sensor because they have many advantages over inorganic materials, especially in terms of their diversity, intrinsic conductivity, fast response, low cost, light weight, ease of synthesis, stability in air, and sensitivity at the room temperature [9-11].
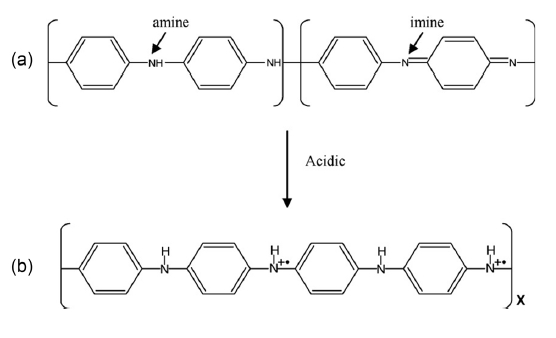
Polyaniline (PANI) is unique among the family of conducting polymers because it is chemically stable and easy to process. It exists as two different emeraldine classes of compounds: an insulating emerald base form (σ ~ 10-5 S/cm), which can be converted into a metallic emeraldine salt conducting form (σ < 103 S/cm) by means of the protonic acid doping process shown in Fig. 1 [12-17]. In addition, polypyrrole (PPy), which has a high level of electrical conductivity with environmental stability, is an extremely useful material for gas-sensing applications [18-20].
Over the last several decades, carbon nanotubes (CNTs) have attracted attention as a promising material that can satisfy the above criteria because they have a high specific surface area for enhanced gas adsorption, excellent electrical properties for high sensitivity, and
chemical stability for resistance to acidic and high-temperature conditions [21-24]. The bottleneck that limits the use of CNTs as a gas-sensing material is their insufficient sensitivity to low concentrations of target gases. In addition, the sensitivity of CNTs is affected by the humidity in the environment because the high specific surface area of CNTs causes the moisture to be adsorbed easily [25,26].
In this study, a PANI/MWCNT composite and a PPy/MWCNT composite were prepared by means of in situ chemical oxidative template polymerization. Gas sensors were fabricated by spin-coating of the conducting polymer-coated MWCNTs.The NH3-sensing properties of conducting polymer-coated MWCNTs were investigated in terms of the electrical resistance changes that occur when gas is adsorbed. This study aims to investigate how the synergistic effects of combining the electrical properties of MWCNTs and the conducting properties of PANI and PPy improve the gas-sensing properties. The conducting polymer-coated MWCNTs are investigated with respect to their morphology, thermal stability, and gas sensitivity.
Aniline monomer (99%), pyrrole monomer (99%), ammonium persulfate (APS), and MWCNTs were purchased from Sigma Aldrich. The diameter of the MWCNTs is between 110 nm and 170 nm, and their purity is higher than 90%. The surfactant sodium dodecyl sulfate (SDS) was obtained from ICN Biomedicals.Hydrochloric acid (HCl) was obtained from Samchun Pure Chemical. Hydrogen peroxide (H2O2) was purchased from Kanto Chemical.
2.2 Synthesis of the PANI/MWCNT composite and the PPy/MWCNT composite
2.2.1 Synthesis of the PANI composite and the PANI/MWCNT composite
PANI was prepared by means of free radical chemical oxidative polymerization through a direct route with HCl as a dopant. A 3 g sample of aniline monomer was dropped into distilled water and stirred continuously for 10 min. A 3.5 mL sample of HCl was added to the aniline solution as a dopant. A 0.8 g sample of APS dissolved in 10 mL of distilled water was slowly added to the aniline solution, and the solution was polymerized for 4 h at 0℃ with constant mechanical stirring. The synthesized PANI was filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The PANI powder was dried in a vacuum at 40℃ for 24 h.
The PANI/MWCNT composites were synthesized by using in situ chemical oxidative polymerization on the MWCNT template. The polymerization of aniline was conducted in distilled water with HCl as the oxidant for aniline and APS as the initiator. A 0.15 g sample of the MWCNTs was dispersed in the surfactant solution and then ultrasonicated for more than 1 h. A 3 g sample of aniline monomer was dropped into the MWCNT solution and stirred continuously for 10 min. A 3.5 mL sample of HCl was added to the reactant solution and then 0.8 g of APS dissolved in 10 mL of distilled water was slowly added to the reactant solution. The polymerization was conducted for 4 h at 0℃ with constant mechanical stirring. The synthesized PANI/MWCNT composites were filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The composite powders were dried in a vacuum at 40℃ for 24 h.
2.2.2 Synthesis of the PPy composite and the PPy/MWCNT composite
PPy was prepared by means of free radical chemical oxidative polymerization through a direct route with H2O2 as the oxidant. A 3 g sample of pyrrole monomer was dropped into distilled water and stirred continuously for 10 min. A 4.5 ml sample of H2O2 was then added to the pyrrole solution. A 0.8 g sample of APS dissolved in 10 mL of distilled water was slowly added to the pyrrole solution and the reactant solution was polymerized for 4 h at 0℃ with constant stirring. The synthesized PPy was filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The PPy powder was dried in a vacuum at 40℃ for 24 h.
PPy/MWCNT composites were synthesized by using in situ chemical oxidative polymerization on the MWCNT template. The polymerization of pyrrole was conducted in distilled water with H2O2 as the oxidant of the pyrrole and APS as the initiator. A 0.15 g sample of MWCNTs was dispersed in the surfactant solution and ultrasonicated for more than 1 h. A 3 g sample of pyrrole monomer was dropped into the MWCNT solution and stirred continuously for 10 min. A 4.5 ml sample of H2O2 was then added to the reactant solution, and 0.8 g of APS dissolved in 10 ml of distilled water was slowly added to the reactant solution.The polymerization was conducted for 4 h at 0℃ with constant stirring. The synthesized PANI/MWCNT composites were filtered and rinsed several times with distilled water, methanol, and acetone, respectively. The composite powders were dried in a vacuum at 40℃ for 24 h.
2.3 Preparation of the gas sensor
The conducting polymer-coated MWCNT samples (0.1 g) were dispersed in acetone (5 g) and sonicated for 30 min so that the samples were uniformly dispersed in the acetone. A sample of the dispersed mixture (0.01 g) was then dropped onto a silicon wafer with an Ovation micro-pipette (VistaLab Technologies, USA) and spin-coated (with an ACE-200 spin coater) at 900 rpm for 4 min. The coated wafer was heated at 40℃ for 10 min to remove the solvent. The thickness of the coating was 15 ㎛ ± 1 ㎛.
2.4 Measurement of the gas sensitivity
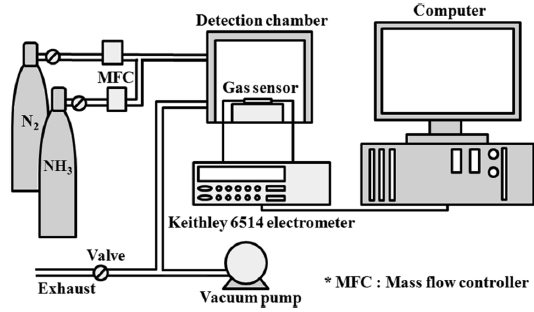
The electrical resistance was measured with a programmable electrometer (Keithley 6514) to evaluate the gas-sensing properties of the samples. This measurement was performed in a stainless steel chamber with a volume of 1500 cm3. The chamber was connected to gas cylinders (NH3, dry air, and wet air with 50% relative humidity). The prepared gas sensor sample was placed in a sealed chamber under vacuum at a pressure of 1 × 10-3 torr. Initially, air was injected into the chamber to stabilize the electrical resistance. The NH3 gas was diluted with air to 1000 ppm. The air was used as a carrier gas for the control of the gas concentrations. A mixture of two different gases was then prepared with a concentration of 50 ppm NH3 in air and injected into the chamber. The total flow rate of the gases was kept constant at 500 sccm. The change in electrical resistance was measured at 298 K ± 1 K. When the electrical resistance was stable during the NH3 gas injection, the injection of NH3 gas was turned off and the recovery of electrical resistance was observed. The gas feeding rate was fixed at 500 sccm in all cases. The sensitivity of the gas sensor (S) is expressed as follows [27,28]:
where R0 is the resistance due to the air and Rg is the resistance measured upon exposure to the NH3 gas. The setup of the gas sensor system is depicted in Fig. 2.
The morphology of the conducting polymer-coated MWCNT samples was characterized by field-emission scanning electron microscopy (FE-SEM). The FE-SEM measurements were taken at 5 kV with an Hitachi S-5500 (Japan). Thermogravimetric analysis (TGA) was performed under a nitrogen flow (50 cm3 min-1) at a heating rate of 10℃ min-1 up to 800℃.
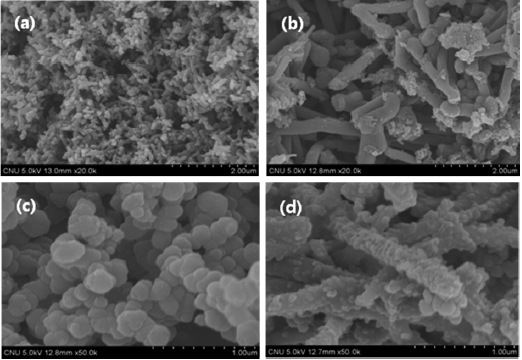
Fig. 3 shows FE-SEM images of PANI, PPy, and the conducting polymer-coated MWCNTs. The rod-like structure of PANI is evident in Fig.3 a. PANI was coated uniformly on the MWCNT templates as shown in Fig.3 b. Fig.3 c shows the granular structure of PPy. Fig.3 d shows that PPy was also coated onto the MWCNT surfaces, which maintained a granular structure.
The bilayered structures of PANI-coated MWCNTs and PPycoated MWCNTs were formed successfully on the outer surface of the MWCNTs. These morphological characteristics are expected to play an important role when the conducting polymercoated MWCNT composites are used as a gas-sensing material.
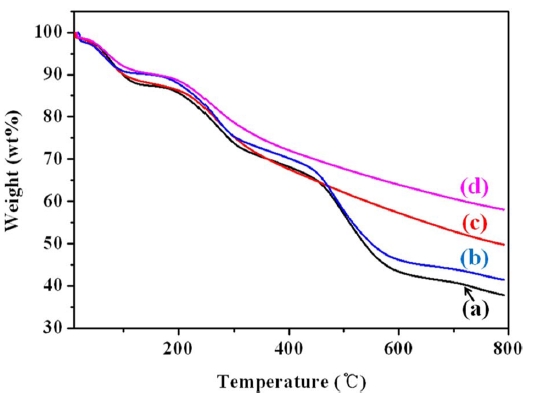
The amount of weight loss and the thermal stability of the
pristine MWCNTs as well as the PANI, PANI/MWCNT, PPy, and PPy/MWCNT composites were determined by means of TGA between temperatures of 25℃ and 800℃. Fig. 4 shows TGA thermograms of the MWCNTs and the PANI, PANI/MWCNT, PPy, and PPY/MWCNT composites. The PANI/MWCNT composite and PPy/MWCNT composite both have better thermal stability than the corresponding conducting polymer alone due to the existence of the thermally stable MWCNTs.
3.3 Gas sensitivity by resistive response
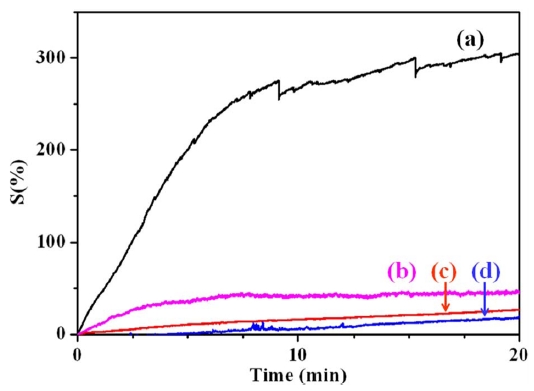
Fig. 5 presents the NH3 gas sensitivity of various conducting polymer/MWCNT composites as measured by changes in the electrical resistance. MWCNTs usually exhibit a change in electrical resistance in accordance with the typical characteristics of a p-type semiconductor. Generally, the electrons travel from CNTs to NH3 gas (reducing gas), resulting in an increase of electrical resistance [8]. The gas sensitivity of pristine MWCNTs is known to be about 200%. In this study, the gas sensitivity of the conducting polymer-coated MWCNTs was evaluated to investigate the synergistic effects of using conducting polymers and MWCNTs. The change in electrical resistance is attributed to the electron charge transfer between NH3 gas and the surface of the conducting polymer/MWCNT composites. PANI has a higher level of sensitivity than PPy because it has a higher level of electrical conductivity than PPy. Accordingly, of all the prepared composite samples, the PANI/MWCNT composite has the highest sensitivity to NH3 gas. The PANI/MWCNT composite has 15 times more sensitivity to NH3 gas than the PPy/MWCNT composite because of the synergistic effect of PANI and the MWCNTs. The conducting polymer-coated MWCNTs efficiently combine the adsorption of gas with a resistive response. This unique effect improves the gas-sensing properties by efficiently changing the level of electrical resistance.
As shown in the FE-SEM images of Fig. 3, the uniform coating of PANI on the MWCNTs forms a thin polymer layer, whereas the aggregation of PPy on the MWCNTs forms a relatively thick, irregular polymer layer on the MWCNTs. This thick irregular layer may hinder the efficient transfer of electrons between NH3 gas and the surface of the conducting polymer/MWCNT composites to induce variation in the resistive response and, consequently, a lower level of gas sensitivity.
Template polymerization was used to prepare composites of conducting polymers and MWCNTs on the surface of MWCNTs to produce an effective gas-sensing material. FE-SEM images confirm that PANI and PPy were both successfully formed on the surface of MWCNTs. The thermal stability of conducting polymer was improved significantly by the formation of composites with MWCNTs. The improved sensitivity to NH3 gas is attributed to the effective electron transfer at the interface of the NH3 gas and the conducting polymer/MWCNT composites; this behavior produces an efficient change in electrical resistance. The gas sensitivity of the PANI-coated MWCNT composite is greatly improved by the uniform coating of PANI on the MWCNTs because of the synergy of efficient adsorption of NH3 gas and the variation in the conduction of electrons.