Kenaf fibers, cellulose-based natural fibers, were used as precursor for preparing kenafbased carbon fibers. The effects of carbonization temperature (700℃ to 1100℃) and chemical pre-treatment (NaOH and NH4Cl) at various concentrations on the thermal change, chemical composition and fiber morphology of kenaf-based carbon fibers were investigated.Remarkable weight loss and longitudinal shrinkage were found to occur during the thermal conversion from kenaf precursor to kenaf-based carbon fiber, depending on the carbonization temperature. It was noted that the alkali pre-treatment of kenaf with NaOH played a role in reducing the weight loss and the longitudinal shrinkage and also in increasing the carbon content of kenaf-based carbon fibers. The number and size of the cells and the fiber diameter were reduced with increasing carbonization temperature. Morphological observations implied that the micrometer-sized cells were combined or fused and then re-organized with the neighboring cells during the carbonization process. By the pre-treatment of kenaf with 10 and 15 wt% NaOH solutions and the subsequent carbonization process, the inner cells completely disappeared through the transverse direction of the kenaf fiber, resulting in the fiber densification. It was noticeable that the alkali pre-treatment of the kenaf fibers prior to carbonization contributed to the forming of kenaf-based carbon fibers.
Kenaf (
There are restrictions in preparing carbon fibers from natural fibers. Natural fibers are not continuous and are non-uniform in fiber diameter. Another critical concern is that cellulose-based fibers, including rayon, experience significant weight loss and thermal shrinkage during carbonization processes [9-11]. In addition, there exist a number of small cells through the transverse direction of cellulose-based natural fibers [12-14] and this may also be one of the restrictions in forming natural fiber-based carbon fibers. Hence, not only reducing the thermal shrinkage in the longitudinal direction and reducing the weight loss, but also obtaining a fiber morphology with the decreased number of cells may be significant issues in preparing a natural fiber-based carbon fiber. Most recently, it has been found that the chemical and morphological characteristics of jute-based carbon fibers depend on the chemical pre-treatment conditions [15]. Sodium hydroxide has been frequently used to modify the surfaces of plant-based natural fibers [16-18] because it plays a significant role in changing fiber morphology, leading not only to somewhat of a disruption of the inner structure of natural fibers with cells but also to remove hemicelluloses components and surface impurities. Ammonium chloride has been used as a flame retardant in preparing rayon-based carbon fibers [19,20]. Therefore it was expected that sodium hydroxide and/or ammonium chloride may contribute to heat-treating and to the preparing of natural fiber-based carbon fibers. Consequently the objective of this study is to diagnose the feasibility of the plant-based natural fiber kenaf as a cellulose-based carbon fiber. Preliminarily,the effects of carbonization temperature (700℃ to 1100℃) and chemical pre-treatment (sodium hydroxide [NaOH] and ammonium chloride [NH4Cl]) at various concentrations on the thermal changes (weight loss in a carbonization furnace, longitudinal shrinkage and thermal stability), chemical composition and fiber morphology of kenaf-based carbon fibers were investigated in the present work.
Kenaf fibers, which were cultivated, extracted and supplied by the Bangladesh Jute Institute, Bangladesh, were used as precursor for preparing kenaf-based carbon fibers. The average length of the kenaf fiber bundles supplied was 70 to 80 mm. The bundles were sufficiently dried at 90℃ for 24 h in a convection oven prior to use.
Prior to each carbonization process, kenaf fiber bundles were chemically pre-treated with NaOH and NH4Cl, purchased from Daejung Chemical Metals Co., Korea. NaOH and NH4Cl solutions with different concentrations were prepared with distilled water. The chemical pre-treatment was conducted by soaking the bundle in 5, 10 and 15 wt% NaOH and NH4Cl solutions for 60 min. After the pre-treatment, kenaf fibers in the bundle were neutralized to reach pH 6.5 by rinsing them with distilled water; they were then dried at 90℃ for 24 h in an oven. Untreated kenaf fibers were also used for comparison.
The untreated kenaf fiber bundles were carbonized at 700℃, 900℃, and 1100℃ with a heating rate of 1℃/min using a tubetype siliconit furnace. The holding time at each carbonization temperature was 60 min. The carbonization furnace was equipped with a mullite tube with an inner diameter of 90 mm, a tube length of 1000 mm, and a heating zone of 250 mm. The furnace was designed to pre-program the heat-treatment profile. Kenaf fiber bundles were freely placed on a graphite plate during the carbonization process. The purging nitrogen (99.999% purity, 150 mL/min) was purged into the tube throughout the carbonization process. The heat-treated kenaf fiber bundles were naturally cooled down to ambient temperature after carbonization. The chemically pre-treated kenaf fibers were heat-treated at 700℃ with a heating rate of 1℃/min. The representative schematic of carbonization equipment was reported elsewhere [21,22].
The weight change of kenaf fibers occurring during the carbonization was examined by means of an analytical scale, which can measure the weight change in sub-milligrams. The longitudinal fiber shrinkage was measured by comparing the change of the pre-marked lengths of each kenaf fiber before and after the carbonization.
An elemental analyzer (EA1108; Fisons, USA) was used to investigate the change of chemical compositions such as carbon, nitrogen and hydrogen atoms before and after each carbonization process. The contents of oxygen atoms in the specimen were estimated by subtracting the C, H, and N contents from the total.
A thermogravimentric analyzer (TGA Q500; TA Instruments, USA) was used to examine the thermal stability of the raw and carbonized kenaf fibers without and with chemical pre-treatment. The weight of each sample was about 20 mg and the measurement was performed at 600℃ with a heating rate of 20℃/min while purging a nitrogen gas (99.9% purity) at a rate of 50 mL/min.
The fracture surface morphology of the raw and carbonized kenaf fibers without and with chemical pre-treatment was observed by means of a scanning electron microscope (SEM, Tescan VEGA II LMU, JEOL JSM-6380, Japan). The cross-section of each fiber was examined after fracturing the samples in a liquid nitrogen bath. All the samples were coated with platinum for 3 min by a sputtering method.
3.1. Carbonization temperature effect on the characteristics of untreated kenaf fibers
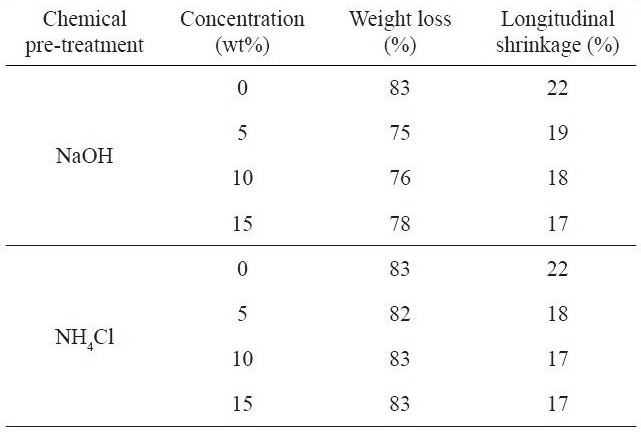
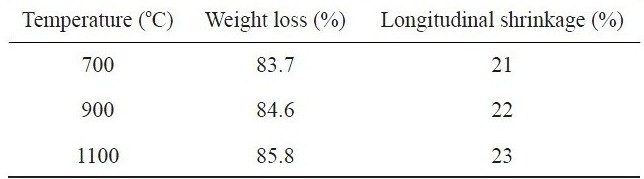
Table 1 summarizes the percent weight loss and longitudinal shrinkage that occurred in kenaf fibers during carbonization at different temperatures. As expected, the weight loss and the longitudinal shrinkage were increased with increasing carbonization temperature from 700℃ to 1100℃. About 86% of the total
Percent weight loss and longitudinal shrinkage of kenaf fibers carbonized at different temperatures
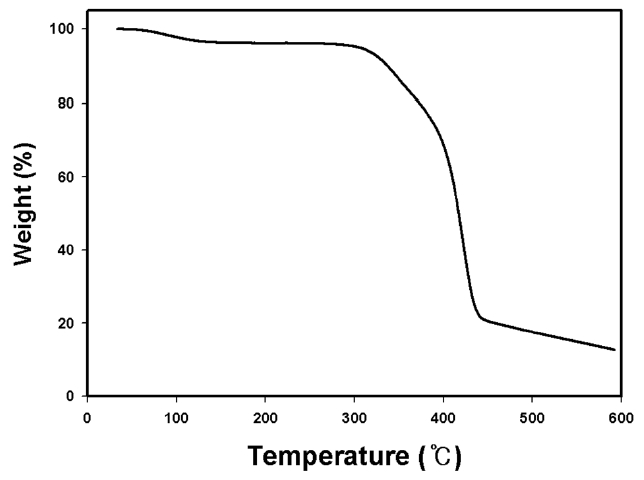
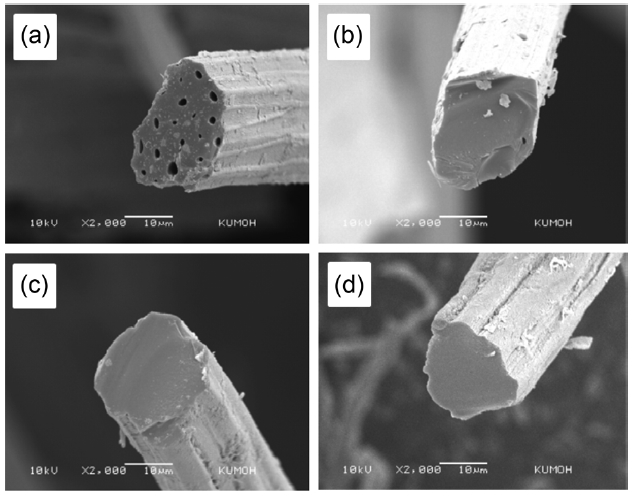
weight of kenaf and about 23% of the initial fiber length were reduced at 1100℃. Such severe changes, similar to those found in cellulose-based carbon fiber precursors like viscous rayon during carbonization, were found to occur because the kenaf fibers were exposed to higher thermal environments with increasing temperature. At elevated temperature, a cellulosic precursor may break down into highly volatile gases and carbon char. During the carbonization process, the disruption of the cellulose molecules can occur with the loss of CO and CO2 and the formation of a structure with higher carbon assay, resulting in the remarkable weight loss. With increasing carbonization temperature the loss of CO and CO2 increases, leading to increased weight loss and longitudinal shrinkage. Fig. 1 shows the thermal stability of raw kenaf measured using TGA. The initial weight loss of raw kenaf below 100℃ was due to the removal of intrinsic moisture contained in the kenaf even after complete drying prior to the measurement. The secondary weight loss occurring at about 320℃ was due to the removal of hemicellulose and low molecular weight components like waxes. The further weight loss that occurred at about 400℃ was due to the thermal degradation of the lignin and cellulose components remaining in the kenaf.
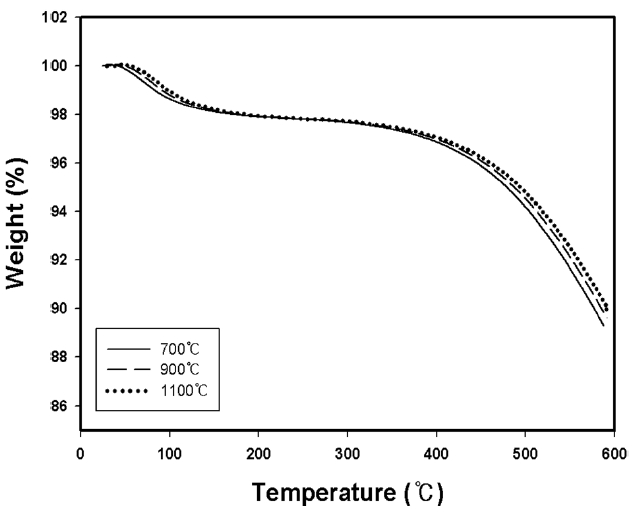
Fig. 2 displays the TGA curves for kenaf fibers carbonized with a heating rate of 1℃/min at 700℃, 900℃ and 1100℃. The thermal stability of carbonized kenaf fiber was slightly increased with the increase of the carbonization temperature. The greater thermal stability of the kenaf fibers exposed to higher carbonization temperature is thought to be because they have more stable structures with higher carbon assay. The residual weight at 600℃ was about 90% of the total. The initial weight loss of about 2% that occurred below 100℃ may be due to the
removal of moisture introduced during the sample preparation. The weight loss of about 8% that occurred above about 400℃ in the carbonized kenaf fibers, as was similarly found in previous work [11] with rayon precursor, was probably due to some uncarbonized chemical moieties existing on the surfaces after the heat-treatment of kenaf fibers, which may have combined with nitrogen molecules flowing during the carbonization process and then have been removed.
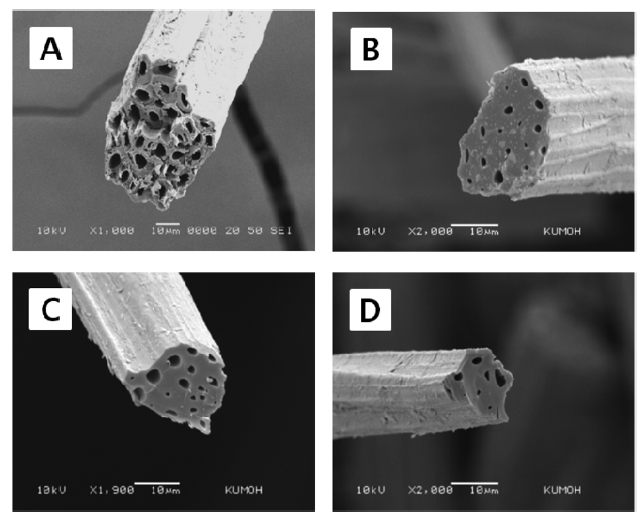
Fig. 3 shows scanning electron micrographs representing the cross-sections of raw kenaf fiber and kenaf fibers carbonized at 700℃, 900℃ and 1100℃. Prior to SEM observations, each fiber was deliberately fractured in a liquid nitrogen bath. As was found for other cellulose-based natural fibers [12-14], raw kenaf fibers have a number of cells in the fiber and their shape is irregular. The size of each cell was in the range of 3 to 7 ㎛. The number and the size of the cells were reduced with the increase of the carbonization temperature from 700℃ to 1100℃, leading to the disruption of the cellulose molecules. Also, the diameter of the cross-section was found to decrease with the increase of the carbonization temperature, as a consequence of the removed volatile gases. The connecting region between the cells was broadened by carbonization. The fiber shape remained irregular. It seemed that each cell was combined or fused with neighboring cells. This phenomenon may have happened as a part of the weight loss and the longitudinal shrinkage with the increase of the carbonization temperature, as was mentioned above.
3.2. Chemical pre-treatment effect on the characteristics of kenaf-based carbon fibers
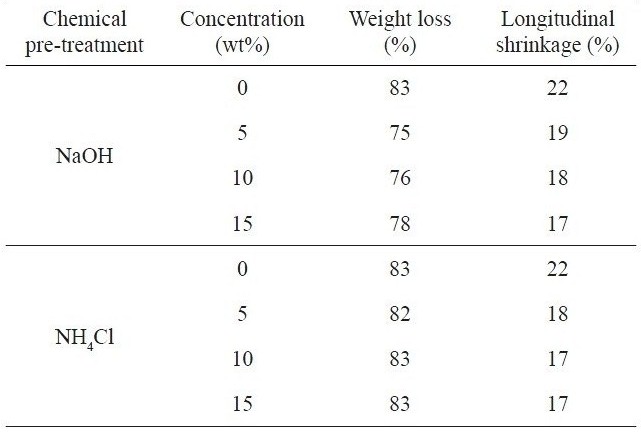
Table 2 presents the percent weight loss and longitudinal shrinkage of kenaf fibers untreated and pre-treated with NaOH and NH4Cl that occurred during the carbonization process at 700℃. The weight loss that occurred in the carbonization furnace decreased from 83% for the untreated kenaf to 75% for the kenaf fiber with 5 wt% NaOH. The weight loss was mainly due to the thermal decomposition of cellulose, lignin and the remaining hemicellulose components making up the kenaf. It has been found that NaOH solution can remove the hemicellulose component in natural fibers like kenaf [13,23]. A higher NaOH concentration may cause a higher removal of hemicellulose and the weight loss can be greater in the carbonization process. The surface impurities existing on the kenaf fiber surfaces were cleaned out during the chemical pre-treatment process. In the case of NaOH pre-treatment, the longitudinal shrinkage of kenaf fiber was slightly decreased from 19% to 17% with the increase of the NaOH concentration. This was due mainly to the removed hemicelluloses and the pre-shrinkage of the kenaf fiber due to the NaOH pre-treatment done prior to carbonization. Such a change may disrupt the pristine kenaf structure, as will be discussed later. The greater removal of the hemicellulose component and the higher pre-shrinkage may result in a decreased longitudinal shrinkage during carbonization, as is indicated in Table 2.
In the case of the NH4Cl pre-treatment, the weight loss (82-83%) was about 5-7% greater than that (75-78%) in the case of NaOH. There was no noticeable pre-treatment effect for
Percent weight loss and longitudinal shrinkge of kenaf fibers with different chemical pre-treatments occurring during carbonization at 700℃
NH4Cl in terms of the weight change that occurred in the carbonization furnace. The hemicellulose component of kenaf was not removed by NH4Cl during the chemical pre-treatment. The marked weight loss was found to result mainly from the thermal decomposition of cellulose, hemicellulose and lignin components of the kenaf fiber. The longitudinal shrinkage was slightly decreased by the pre-treatment with NH4Cl, compared with that of the sample pre-treated with NaOH. This may be evidence of a thermal retardation effect of NH4Cl, which can also be used as a retarding agent for thermal decomposition, particularly in preparing rayon-based carbon fibers [19]. The variation of NH4Cl concentration did not significantly change the weight loss and the longitudinal shrinkage. In spite of the role of thermal retardation of NH4Cl, the reduced percentage weight loss indicated that alkali pre-treatment with NaOH could be more useful than with NH4Cl for reducing the weight loss of kenaf in the carbonization furnace and also to increase the product yield of kenafbased carbon fibers.
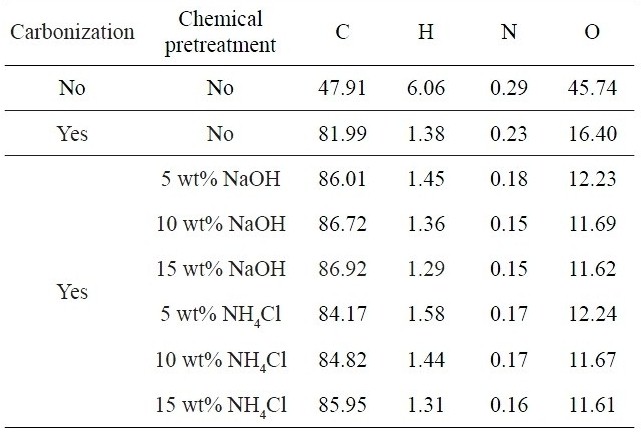
Table 3 compares the chemical compositions measured for kenaf fibers without and with carbonization at 700℃ in the absence and presence of chemical pre-treatment. Raw kenaf fibers exhibited carbon contents of about 48% and the contents were greatly increased to 82% after carbonization and further increased by 2-5% even after the chemical pre-treatment. On the other hand, the contents of hydrogen, nitrogen and oxygen atoms were found to decrease. After the pre-treatment, the O and N contents were further decreased, whereas the H contents did not depend on the pre-treatment. The C contents were slightly increased with increasing NaOH concentration, whereas the O contents were decreased as well. It was noted that the chemical pre-treatment played a positive role in increasing the carbon yield of kenaf-based carbon fiber.
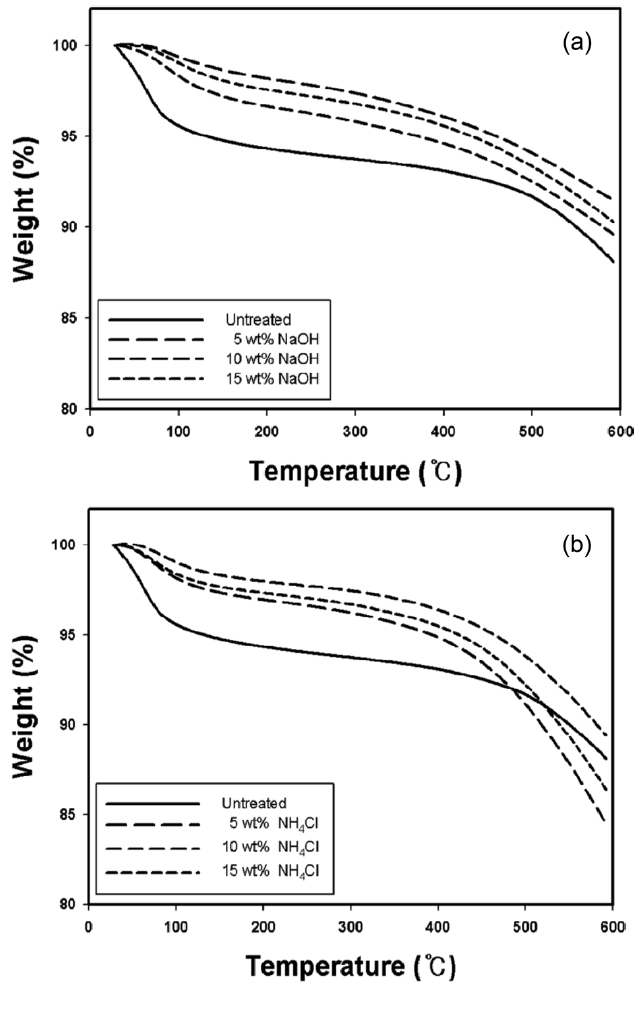
Fig. 4 shows thermal stability of kenaf fibers carbonized at 700℃ in the absence and presence of chemical pre-treatment. The TGA curves inform us that the carbonized kenaf fibers with chemical pre-treatment exhibited greater thermal stability than those without pre-treatment, especially below about 500℃; also, the greatest thermal stability was obtained with the carbonized kenaf fiber pre-treated with 15 wt% NaOH. The small initial weight drop that occurred below 100℃ may be due to
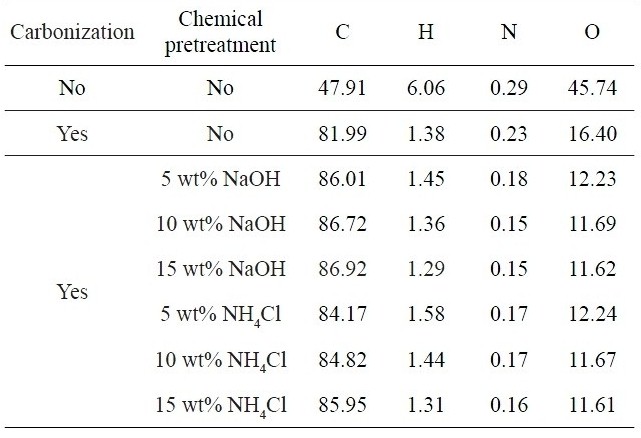
Chemical compositions measured for kenaf fibers without and with carbonization at 700℃ in the absence and presence of chemical pre-treatment
the removal of moisture introduced during the sample preparation. The further weight drop of the carbonized kenaf fibers is probably explained by the fact that some un-carbonized chemical moieties existing on the surfaces after the heat-treatment of kenaf fibers may be combined with nitrogen molecules flowing during the carbonization process and then removed, as was described above.
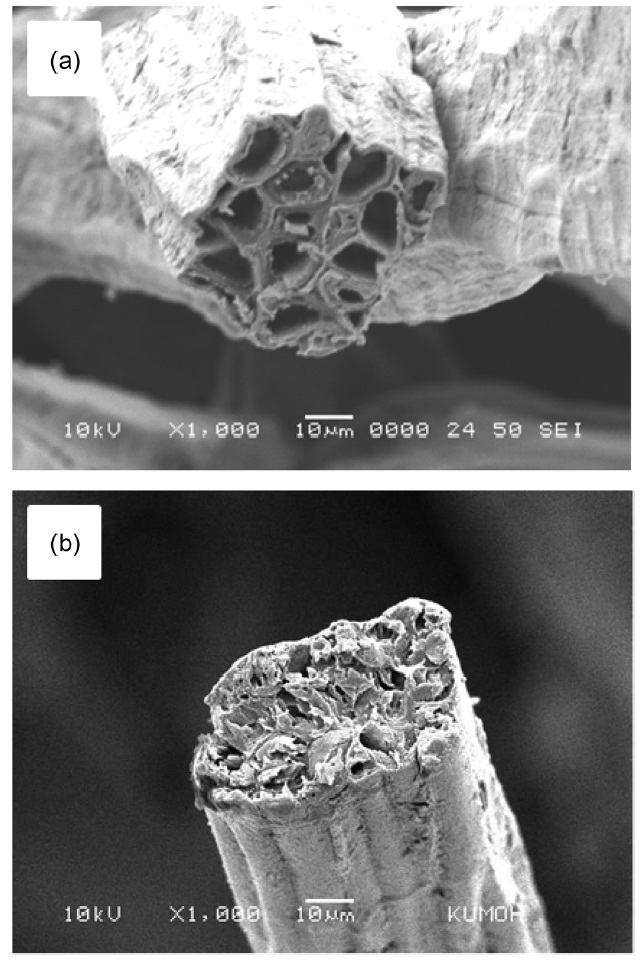
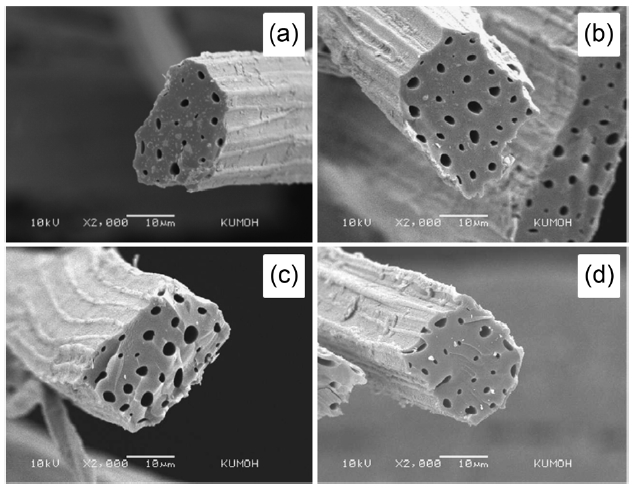
Fig. 5 displays SEM micrographs representing the fractured cross-sections of raw and 15 wt% NaOH-treated kenaf fibers. The fractured surfaces were not clearly cut because raw and untreated kenaf fibers without heat-treatment were somewhat ductile. As expected, raw (no pre-treatment and no carbonization) kenaf fibers exhibited a number of micrometer-sized cells, as can be seen in Fig.3 a. It was found that the cross-section pattern was changed distinguishably. It is noticeable that the cells were not visible in Fig.5 b. While the hemicellulose component was removed by the pre-treatment with NaOH, it is likely that the cellulose and lignin components remaining in the kenaf may have been re-arranged. The struts or cell walls existing between the cells were destroyed and combined with the neighboring cells. However, no mechanism or detailed explanation has been proposed.
Fig. 6 shows the fiber morphology representing the fractured cross-sections of kenaf fibers untreated and pre-treated at different NaOH concentrations and then carbonized at 700℃. The crosssection pattern was flat-cut after carbonization. This means that the fiber surfaces became quite brittle. The fiber shape remained irregular, as was previously found in studies of rayon-based carbon fibers [24]. After the pre-treatment and carbonization at 700℃, it can be said that the average fiber diameter of kenafbased carbon fiber was in the range of 15 to 25 ㎛, depending on the circumferential shape. Comparing with the image shown in Fig.5 a, it can be clearly observed that the inner structure, including the struts or cell walls between the micrometer-sized cells of the kenaf fiber, became dense, exhibiting smaller cells with different sizes. It is likely that the cells were combined or fused and then re-organized with neighboring cells and cell walls, showing a lower number of cells and overall smaller cell size. It turns out that molecular or cellulosic re-organization took place in the inner structure during the carbonization process. After the NaOH pre-treatment, the cross-section pattern was totally changed, indicating that it became denser than that of the untreated sample.Also, the inner cells almost disappeared at 5 wt% NaOH and completely disappeared at 10 and 15 wt% NaOH through the transverse direction of the kenaf, as was found with jute-based
carbon fibers in the previous report [15].
Fig. 7 shows the fiber morphology, representing the fractured cross-sections of kenaf fibers untreated and pre-treated at different NH4Cl concentrations and then carbonized at 700℃. As was observed in the case of NaOH pre-treatment, the fiber crosssection was flat-cut and irregular, reflecting the brittle fracture surfaces. Unlike NaOH pre-treated and then carbonized kenaf fibers, kenaf samples that were pre-treated at different NH4Cl concentrations and then carbonized showed a number of microsized cells that were easy to observe through the transverse direction. In addition, the pre-treatment of kenaf with NH4Cl did not change the fiber morphology significantly in the absence of the carbonization process, unlike the result found in the case of NaOH pre-treatment. It may be concluded, in terms of the fiber morphology as well as the thermal changes, that the alkali pretreatment of kenaf with NaOH of 10 to 15 wt% prior to carbonization contributed to forming kenaf-based carbon fibers.
1. Weight loss and longitudinal shrinkage of kenaf fibers occurred in the carbonization furnace and the thermal stability was increased with the increase of the carbonization temperature from 700℃ to 1100℃.
2. NaOH pre-treatment was useful for reducing the weight loss and the longitudinal shrinkage of kenaf fibers in the carbonization furnace and also for increasing the product yield of kenaf-based carbon fibers. A higher NaOH concentration may cause a higher removal of hemicellulose and, as a result, a greater weight loss after carbonization. Pre-treatment with NH4Cl did not play a role in the weight loss. The carbonized kenaf fibers with chemical pre-treatment exhibited greater thermal stability than those without the pre-treatment.
3. The carbon content of raw kenaf fibers was greatly increased from 48% to 82% after carbonization and further increased about 2-5% in the presence of chemical pre-treatment.
4. The inner structure, including the struts or cell walls between the micrometer-sized cells of kenaf fiber, became dense.It seems that the cells were combined or fused and then re-organized with neighboring cells and cell walls, showing a lower number of cells and a smaller cell size. After NaOH pre-treatment, the cross-section pattern was totally changed, becoming denser than that of the untreated sample. Also, the inner cells completely disappeared at 10 and 15 wt% NaOH through the transverse direction of the kenaf.
It may be concluded that in terms of fiber morphology as well as of thermal changes and increased carbon content, the alkali pre-treatment of kenaf with NaOH of 10 to 15 wt% prior to carbonization contributed to forming kenaf-based carbon fibers.