Various silanes, amino silane, vinyl silane, TESPD, and ZS (TESPD/zinc soap complex), are added into chlorinated isobutylene-isoprene copolymer (CIIR)/soft clay/carbon black (CB). The vulcanization characteristics, the processability, and the mechanical properties are measured. In soft clay/CB filled CIIR system, there are no significant changes in Mooney viscosity among compounds. Vinyl silane added compound shows a low extrusion torque. All the silane added compounds shows an increased modulus. The mechanical properties are significantly increased when the S2 is added into CIIR/soft clay/CB compounds.
Carbon black particles have been used for filler and antistatic filler for a long time in rubber industry due to good reinforcing property and electrical conductivity when they are added into elastomers[1,2]. Carbon blacks have been added into various rubbers such as natural rubber[3-6], styrene-butadiene rubber[7-12], etc. Recently carbon nanotubes, which first discovered by Ijima in 1991[13], are appeared as a candidate for replacing carbon black in the non-black tire application[14-24]. Carbon nanotubes are good electrical conductive materials[24]. Addition of processing additives (soap-, fatty acid ester-, organo silicon-, octadecyl amine compound) improves the processability of the compound[15-24]; however, at large dosage, they do not increase the mechanical properties due to increased slip interface between polymer chains and polymer-filler.
We have shown in Part I that the effects of various silanes, amino silane, vinyl silane, bifunctional organo silane (TESPD), and ZS, on hard clay (Suprex)/carbon black filled chlorobutyl rubber (CIIR) for innerliner application with respect to the vulcanization properties, the processability, and the physical properties of the compounds.
In this research, we investigate various silane effects on soft clay (GK990)/carbon black filled CIIR compounds at the same condition as applied on Part I. The rheological properties and mechanical properties are measured.
The silanes used in this study were amino (SCA-1100), vinyl (SCA-972), and sulfur (SCA985 bis(triethoxysilylpropyl) disulfide (TESPD) (S2)), which are obtained from Struktol. The elastomer used was chlorobutylene-isoprene rubber (CIIR), which was supplied by an Exxon Chemical the brand name of CB1066. The filler used was N774 (SRF-HM NS), which has BET 29 (m2/g) product of Cabot. Various additives, including activators (ZnO, stearic acid), Maglite K (magnesium oxide, scorch retarder, activator, vulcanizer, acid acceptor, stabilizer, UV screener), processing aid, plasticizer, dispersing aid, (Circosol 4240), homogenizer (40MS, aromatic and aliphatic hydrocarbon chain mixture), extender GK990 (hydrous aluminum silicate, soft clay) (GK), median particle diameter 1.3 ㎛, BET 17 (m2/g)), Allied whiting (calcium carbonate, mean particle size 15.5 ㎛), curing agent (Sulfur), and accelerator (MBTS (2,2’-dithiobisbenzothiazole)), were used for general rubber compound. The information on the materials used in the study is summarized in Table 1.
2.2. Processing and Characterization
In order to compare with hard clay filled system, in this study we used the same mixing and testing condition with hard clay/carbon black filled CIIR system as shown in Part I (e.g. mixing, Mooney
[Table 1.] Materials used in this study
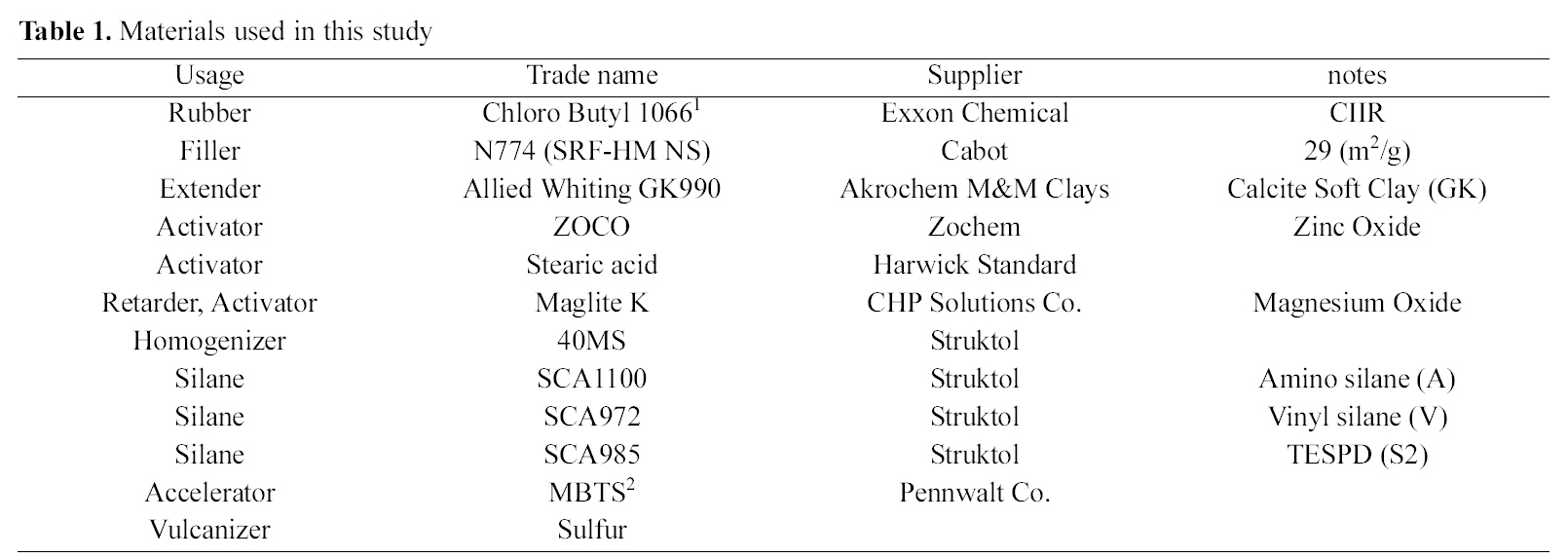
Materials used in this study
[Table 2.] Mixing formulations and procedure on CIIR compounds Formulation
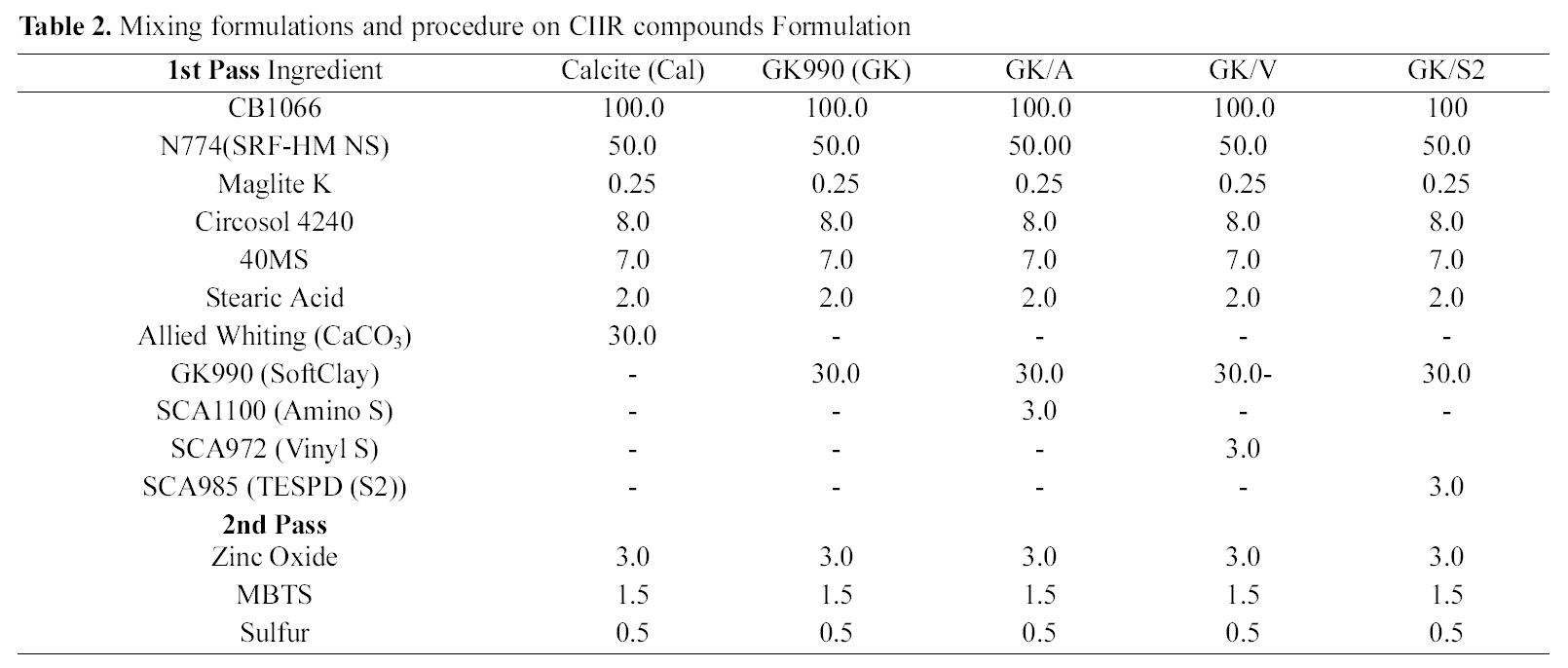
Mixing formulations and procedure on CIIR compounds Formulation
Mixing Procedure
1st stage mixing (Master batch mixing (MB1)): Fill factor 0.8, 60 rpm and 30 psi Starting Mixer Temp: 70℃ (158℉)
1. Add rubber
2. Mix for 90 sec
3. Add CB and other additives
4. Mix for 150 sec and sweep.
5. Sweep at 210 sec
6. Drop at 300 sec (or 140℃/284℉)
2nd stage mixing (MB2): 60 rpm and 30 psi Starting Mixer Temp: 65℃ (150℉)
1. Add MB1 and curatives
2. Drop at 120 sec (or 100℃ (212℉)).
viscosity measurement, cure rheometer test, extrusion, tensile test, tear resistance test, and fatigue to failure tester tests). The mixing formulations and procedures are summarized in Table 2.
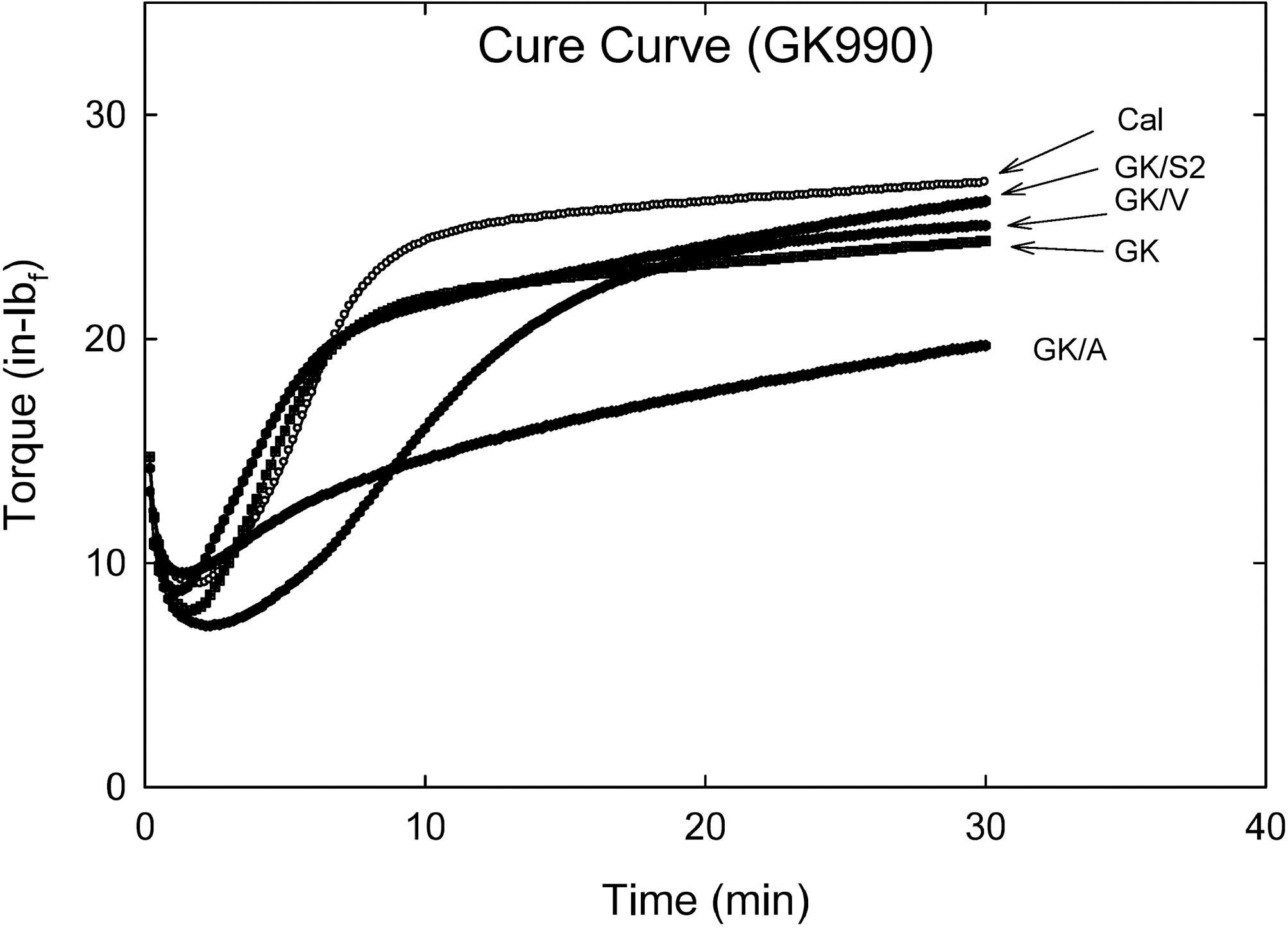
Fig. 1 shows vulcanization curves of various silane (Amino, Vinyl, and S2 (TESPD)) treated soft clay (GK990) filled in CIIR matrix for innerliner compound. Addition of amino silane and S2 to GK did not lower the torque minimum (ML), however, the vinyl silane added compound showed a significant reduction in ML.
As the vulcanization time was increased, the torque level of each compound was changed. Among them, amino silane added compound showed a marching behavior, while vinyl silane added one showed a delay scorch behavior with ‘∫’ shape.
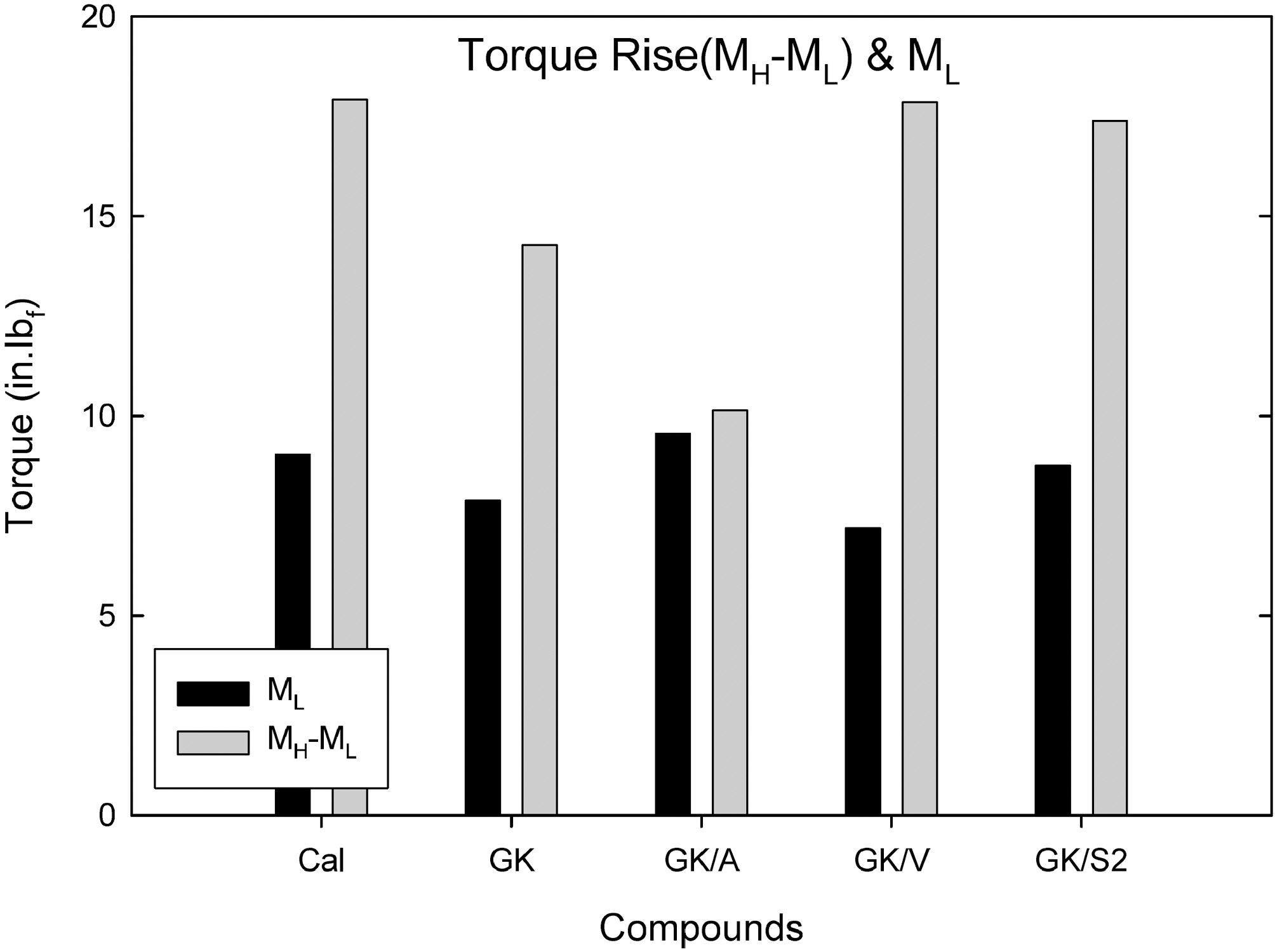
Fig. 2 shows torque rise (MH-ML) and torque minimum (ML) measured from cure rheometer at 100℃ for 30 min. Addition of vinyl and S2 silane increased the torque rise, and amino silane added compound showed a significant reduction in torque rise.
Addition of amino- and S2 silanes to GK slightly increased the torque minimum (ML), and vinly silane added compound showed a reduction in ML.
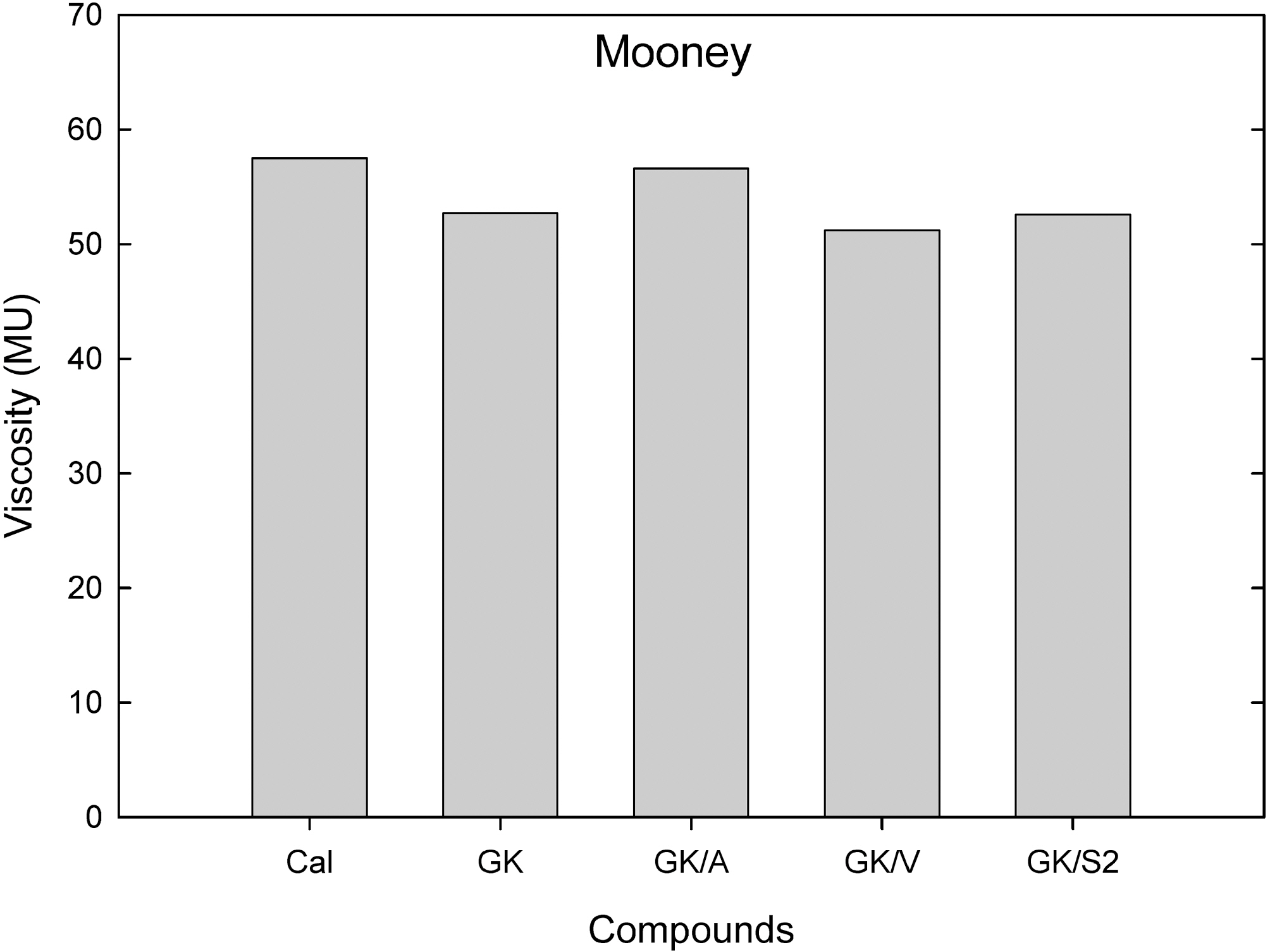
Fig. 3 represents Mooney viscosity (ML1+4) measured from Mooney viscometer at 100℃. Addition of the vinyland S2 silanes did not lower the ML, while that of the amino silane added one showed a slightly increased viscosity.
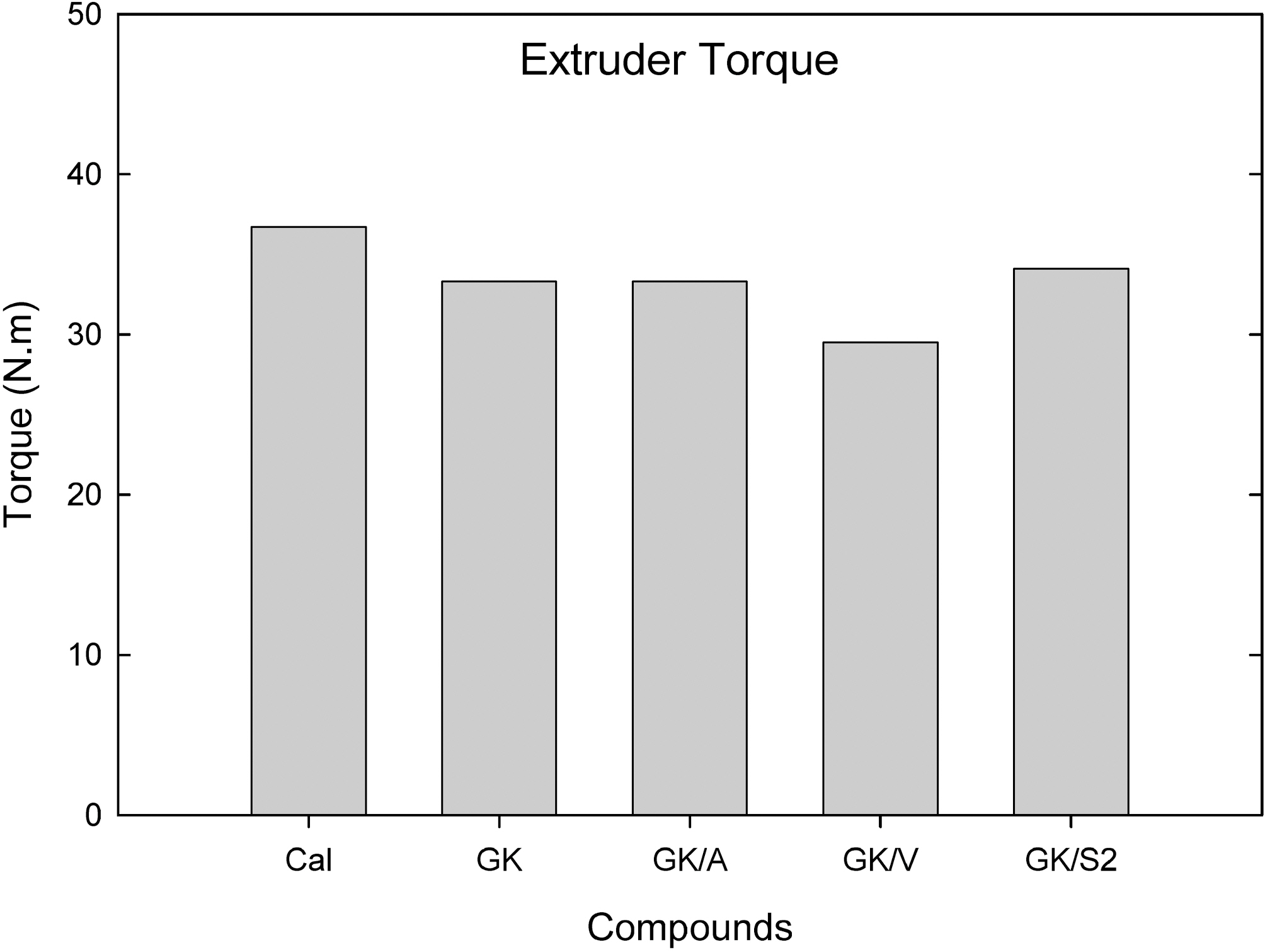
Fig. 4 shows extruder torque buildup on the screw surface measured from a single screw extruder at 100℃ and 25 rpm. Addition of amino and S2 silanes to GK did not show significant changes in extruder torque; however, vinyl silane added compounds showed some reduction in torque buildup on the screw surface.
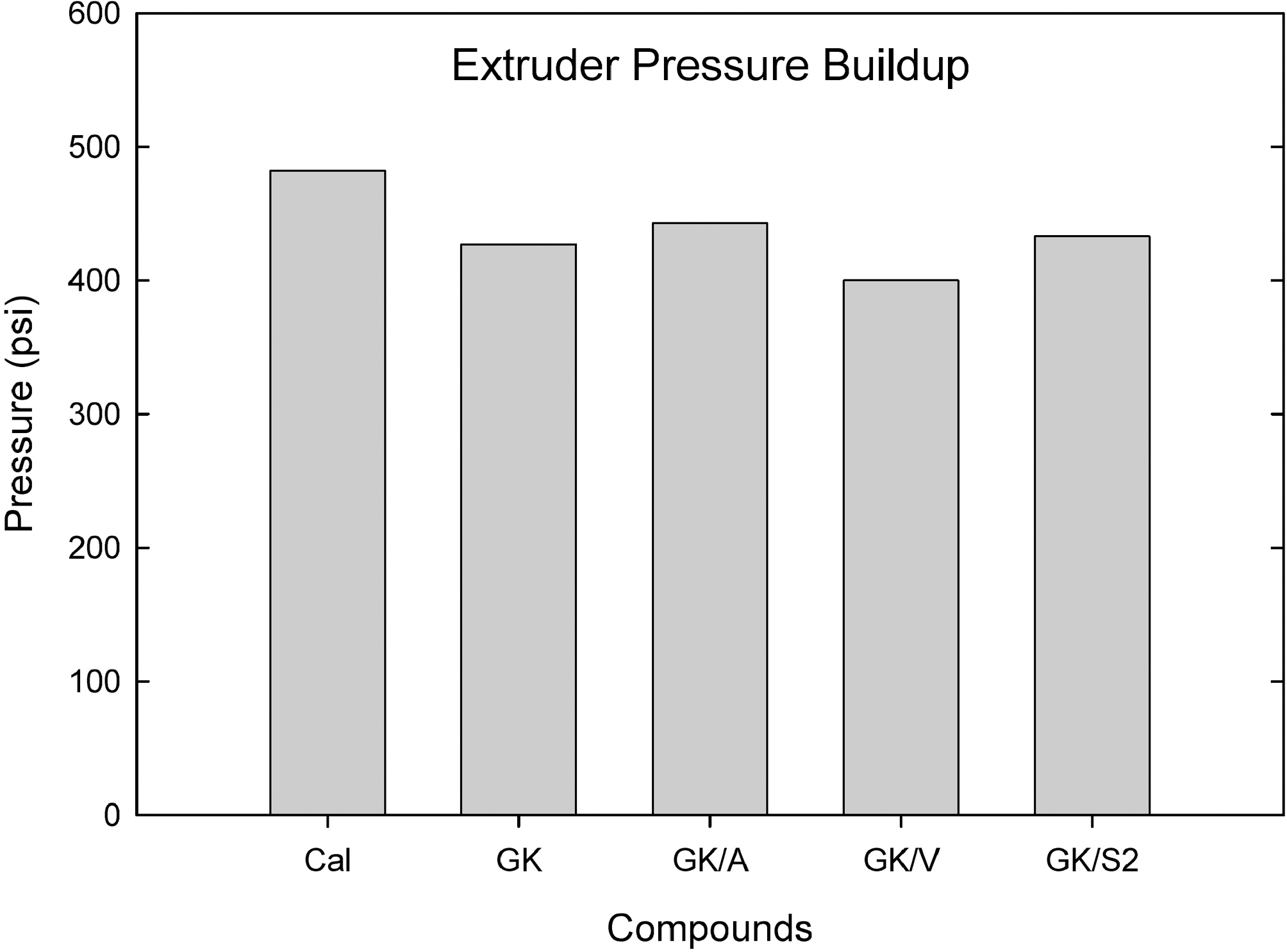
Fig. 5 shows an extruder pressure buildup in the extruder barrel measured from a single screw extruder at 100℃ and 25 rpm passed through a Garvey die. Addition of amino- and S2 silanes to GK increased the pressure buildup in the extruder die end, and
vinyl silane added compounds showed a slight reduction.
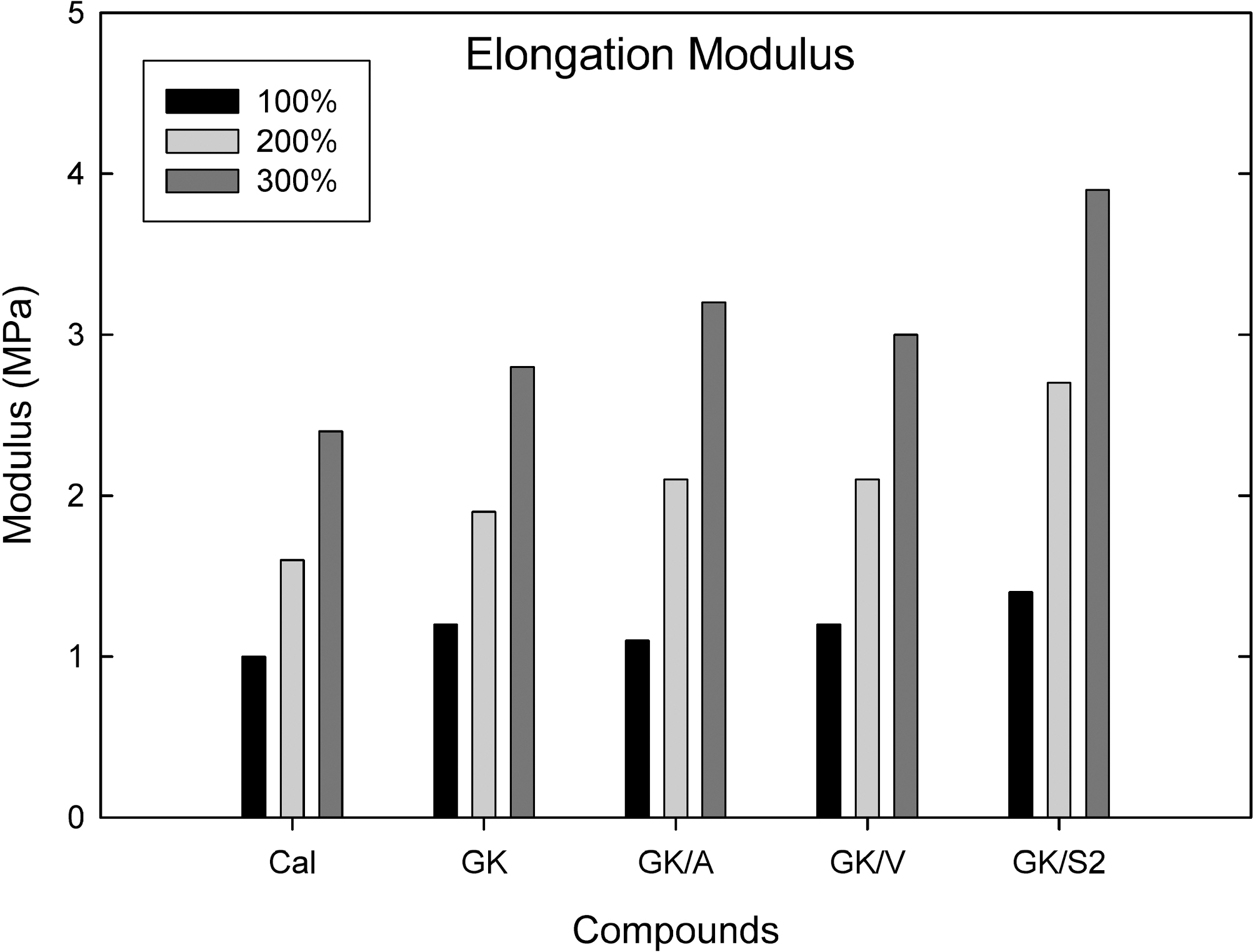
Fig. 6 shows an elongation modulus measured from an Instron tensile tester at 100, 200, and 300% elongation. The GK filled compound showed a higher elongation modulus than calcite filled compound. Addition of silanes (amino, vinyl, and S2) to GK increased the elongation modulus. Among them S2 added compound showed a significant
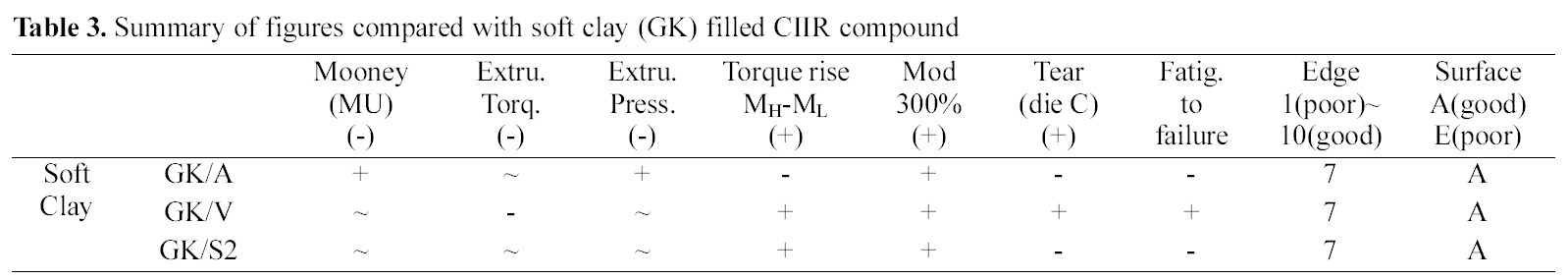
[Table 3.] Summary of figures compared with soft clay (GK) filled CIIR compound
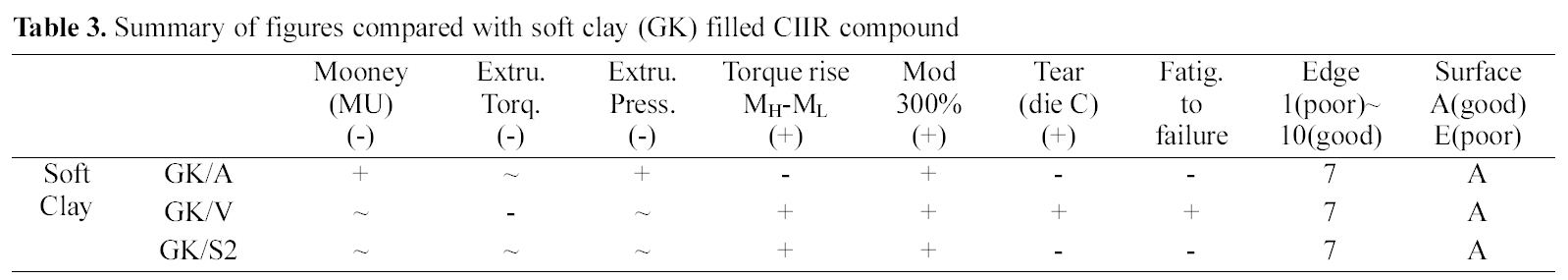
Summary of figures compared with soft clay (GK) filled CIIR compound
increase in elongation modulus; however, vinyl silane added compound showed a slight increase in elongation modulus.
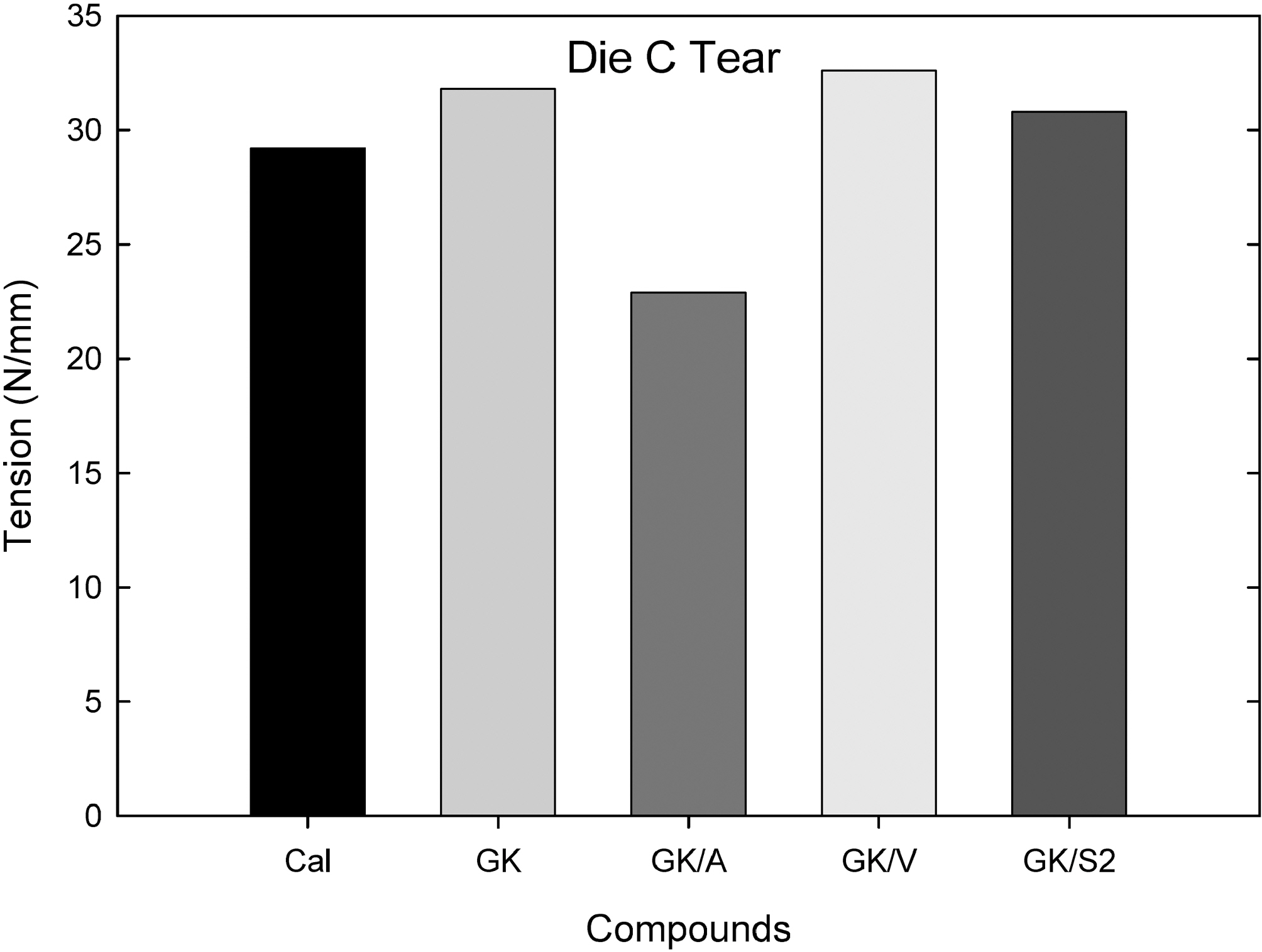
Fig. 7 shows a die C tear tension (N/mm) measured from an Instron tensile tester. Addition of the vinyl silane to GK slightly increased the elongation modulus and the S2 silane added one did not show significant changes; however, the amino silane added compound showed a significant decrease in tear tension.
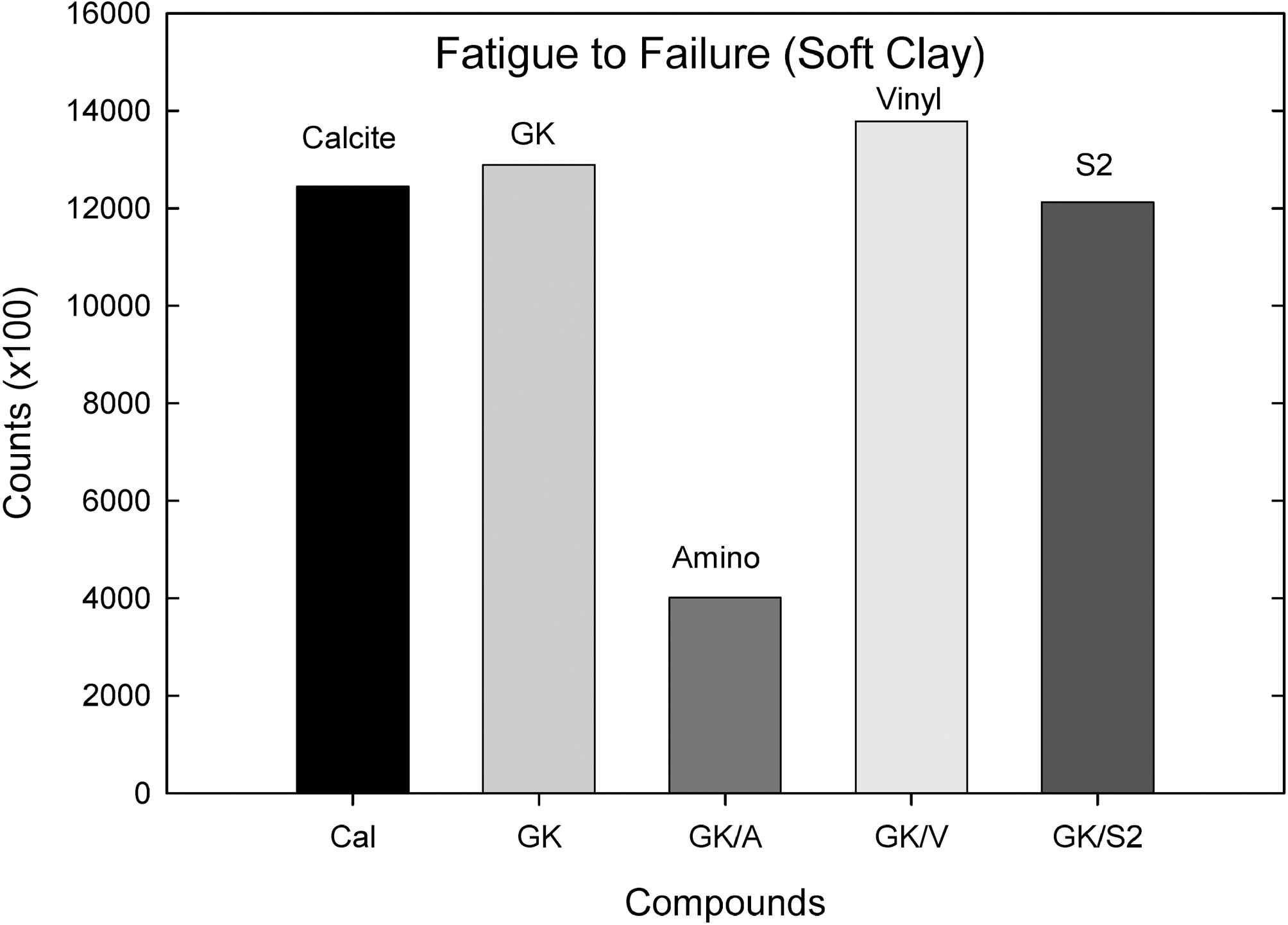
Fig. 8 shows a fatigue to failure test. Addition of the vinyl silane to GK slightly increased the counts and the S2 silane added one did not show significant changes; however, the amino silane added compound showed a significant decrease in counts.
Overall, extrusion torque was increased by the addition of vinyl silane while other silane added ones did not show significant differences. Elongation modulus properties were increased by the addition of silanes (amino, vinyl, and S2). In tear tension measurement, the amino- and S2 silane added compounds showed a lower tension than the GK, while the vinyl-, and the S2 silane added one showed an increased tear tension. Among them the vinyl silane added compound showed reduced extruder torque and increased mechanical properties in soft clay filled compounds as shown in Table 3.
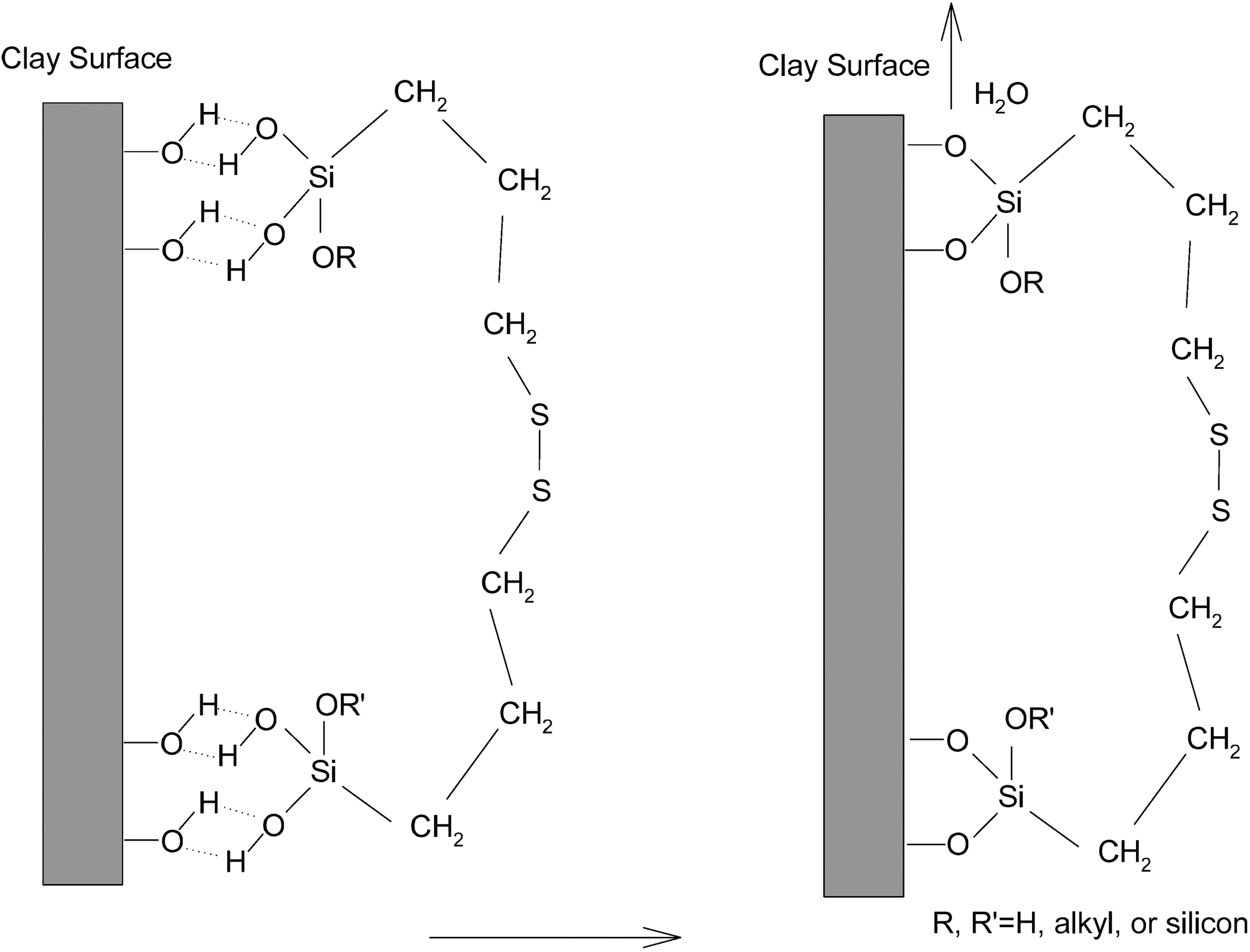
It seems that the S2 effectively coupled with double bonds in the CIIR and on the soft clay surface resulted in a
formation of a strong 3-dimensional network structure between CIIR and soft clay as shown in Fig. 9 and 10. One end of silane (alkoxy group) coupled with soft clay surface via hydrolysis reaction (Fig. 9) and the other end of silane (sulfur group) chemically coupled with double bond in the CIIR chain (Fig. 10).
The vinyl silane added compounds showed both a better processability and mechanical properties compared to the S2 ones at low concentration. It seems that vinyl silane not only couples on a soft clay surface, but also chemically reacts with free sulfur forming a covalent bond with CIIR, which leads to a strong 3-dimensional network structure between CIIR and soft clay. It seems coating on the extruder surface inducing slip between screw surface and the compounds, which results in a reduced extrusion torque.
Various silanes (amino, vinyl, and S2) were added into CIIR/soft clay (GK)/CB compound and their processabilities and mechanical properties were compared. It is concluded as followed.
Addition of silanes (amino, vinyl, and S2) increased elongation modulus. Only vinyl silane added compound showed a slightly better extrusion torque, elongation modulus and fatigue to failure counts while other silane added ones did not show significant improvements. The addition of S2 (TESPD) into the compounds increased mechanical properties.