Hydrogels are defined as the polymeric networks capable of imbibing and retaining large quantities of water in their swollen structures without dissolution or loss of their three dimensional network structure. Smart hydrogels are able to alter their volume and properties in response to environmental stimuli such as pH, temperature, ionic strength, and electric field. Drug delivery systems based on the smart hydrogels have many advantages that overcome the limitation of the conventional polymeric systems [1-5]. Because of their drastic response to environmental stimuli, these hydrogels have been investigated for many biomedical and pharmaceutical applications including controlled drug delivery, molecular separation, tissue culture substrates, enzyme activity controlling systems, and materials for improved biocompatibility [6-12].
Poly(acrylic acid) (PAAc) is extensively studied as a pHsensitive polymer. When the pH is higher than the pKa of PAAc, PAAc hydrogel would swell due to the repulsion among carboxylate anions. Several types of pH-sensitive hydrogels have also been developed [13, 14].
Activated carbon (AC) is one of the most effective adsorbents for organic compounds because of their extended surface area, high adsorption capacity, microporous structure and special surface reactivity [15, 16]. Coconut shell-based activated carbon has mainly micro-pores to meso-pores whereas coal-based activated carbon has mainly meso-pores and macro-pores.
The purpose of this study was to investigate the possibility of AC as effective drug reservoir for PVA/PAAc hydrogel system. AC-containing PVA/PAAc composite hydrogels were prepared by free-radical polymerization. The effect of AC on the drug release behavior from PVA/PAAc hydrogels was studied in terms of pH-sensitivity.
Coal-based activated carbon and coconut-based activated carbon were purchased from Dong Yang Tanso Co. (Korea). Before using them, washing process was carried out at 100℃ for 2 h using mixed acids of sulfuric (35 wt%) and nitric (20 wt%) acids with weight ratio (1:1). Poly(vinyl alcohol) (PVA), acrylic acid (AAc), glutaraldehyde (GA), ethylene glycol dimethacrylate (EGDMA) and potassium persulfate (KPS) used in this study were obtained from Sigma Chemical Company. Coomassie brilliant blue R-250, the model drug, was obtained from Bio-rad.
2.2. Synthesis of PVA/PAAc composite hydrogels containing AC
The PVA/PAAc interpenetrating polymer network (IPN) hydrogels were synthesized by the free radical polymerization. The polymerization was carried out in de-ionized water using GA and EGDMA as a crosslinker of PVA and PAAc, respectively. KPS was used as an initiator. They were dissolved to form the aqueous solution with dispersed AC. The reaction mixture was stirred for 30 min at room temperature. Subsequently, nitrogen was bubbled through the mixture for 20 min to remove the oxygen dissolved in the reaction mixture. And then, the mixture was reacted by stirring for 2 hr at 70℃ to prepare AC-containing PVA/PAAc composite hydrogel. These samples were washed with distilled water at room temperature by replacing with fresh distilled water every few hours. AC-containing PVA/PAAc composite hydrogel was cut into disc-like pieces having approximately 10 mm in diameter and 5 mm in thickness for the following studies. Swollen hydrogel disks were dried in vacuum oven.
2.3. Characterization of PVA/PAAc composite hydrogels containing AC
2.3.1. Swelling behavior
Different pH buffers (2, 7 and 10) were used as swelling media and the swelling behaviors were determined gravimetrically. The dried samples were immersed into the different pH buffer solutions for a certain period of time until the swelling equilibrium was reached and then these hydrogels were taken out, wiped with tissue paper to remove excess of buffer solution on the surface of hydrogel, and weighed immediately. The equilibrium swelling (%) of the hydrogels was calculated follows:
Swelling (%) = (Ws-Wd)/Wd×100,
where Wd and Ws are the weights of dry and swollen samples, respectively.
2.3.2. SEM
The morphology of samples was investigated using UHR FE-SEM (ultra-high resolution field emission - scanning electron microscope, Hitachi, S-5500) apparatus. As a pretreatment, samples were vacuumed up to 10-3 Pa and sputtered using Pt. SEM images were obtained at 300 and 1000 resolutions.
2.3.3. BET analysis
In order to investigate the pore structure such as specific surface area, pore volume, and micropore fraction, BET apparatus (ASAP 2020) was used. Every sample was degassed up to 10-3 Pa and then, nitrogen gas (99.9999%) adsorption was carried out at 77 K. Specific surface area (S.S.A.) was calculated by using BET equation and the fraction of micropore surface area was measured by HK (Horvath-Kawazoe) method.
2.3.4. Release behavior
Drug release was carried out in pH 2, 7 and 10 buffer solutions. The cumulative amount of released drug was measured by using a UV spectrometer (Optizen 2120 UV, Mecasys, Korea), and a peak search was carried out in order to choose the wavelength range. At each point, the buffer solution was removed and replaced with fresh one to reduce the experimental error. As a reference, the drug release experiment using only AC was carried out. All release studies were carried out in triplicate. The results were presented in terms of cumulative release as a function of time:
Cumulative amount released (%) = (Mt/M) × 100
where Mt is the amount of drug released from the composite hydrogel containing AC at time t and M is the amount of coomassie brilliant blue pre-loaded in AC.
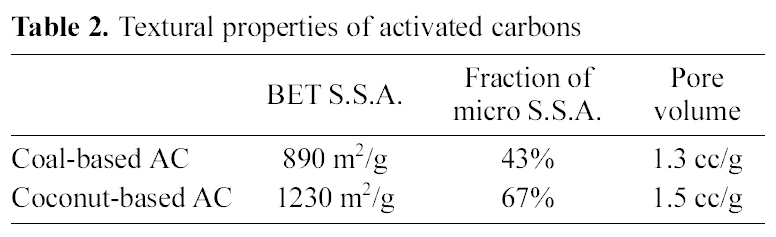
The textural properties of two kinds of AC are presented in Table 2. The BET S.S.A. of coal-based and coconut-based ACs was 890 and 1230 m2/g, respectively. The fraction of micropore structure was 43 and 67% and the total pore
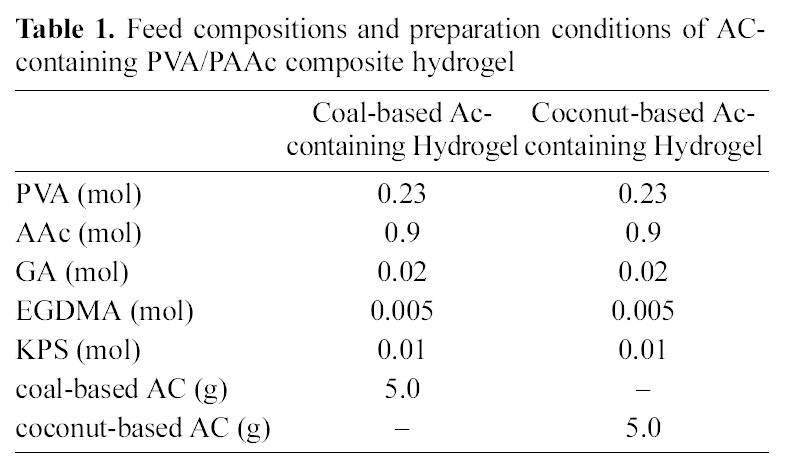
[Table 1.] Feed compositions and preparation conditions of AC-containing PVA/PAAc composite hydrogel
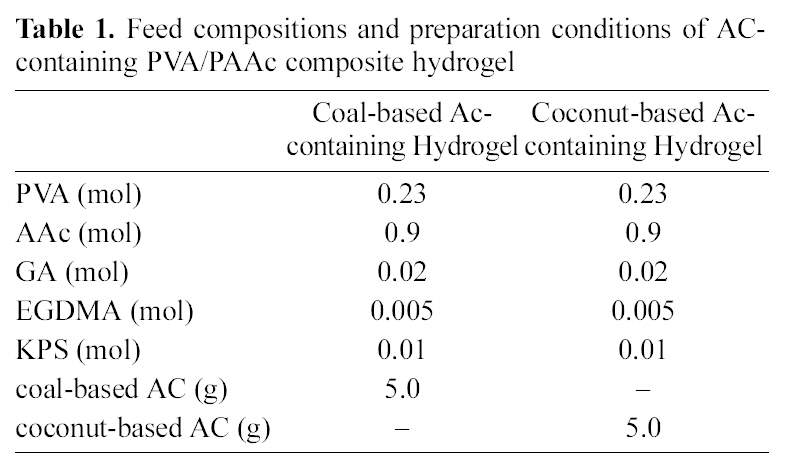
Feed compositions and preparation conditions of AC-containing PVA/PAAc composite hydrogel
volume was 1.3 and 1.5 cc/g, respectively. This highly developed pore structure contributed to the efficient loading and releasing of the drug due to the excellent adsorbing properties.
3.2. SEM images of PVA/PAAc composite hydrogels containing AC
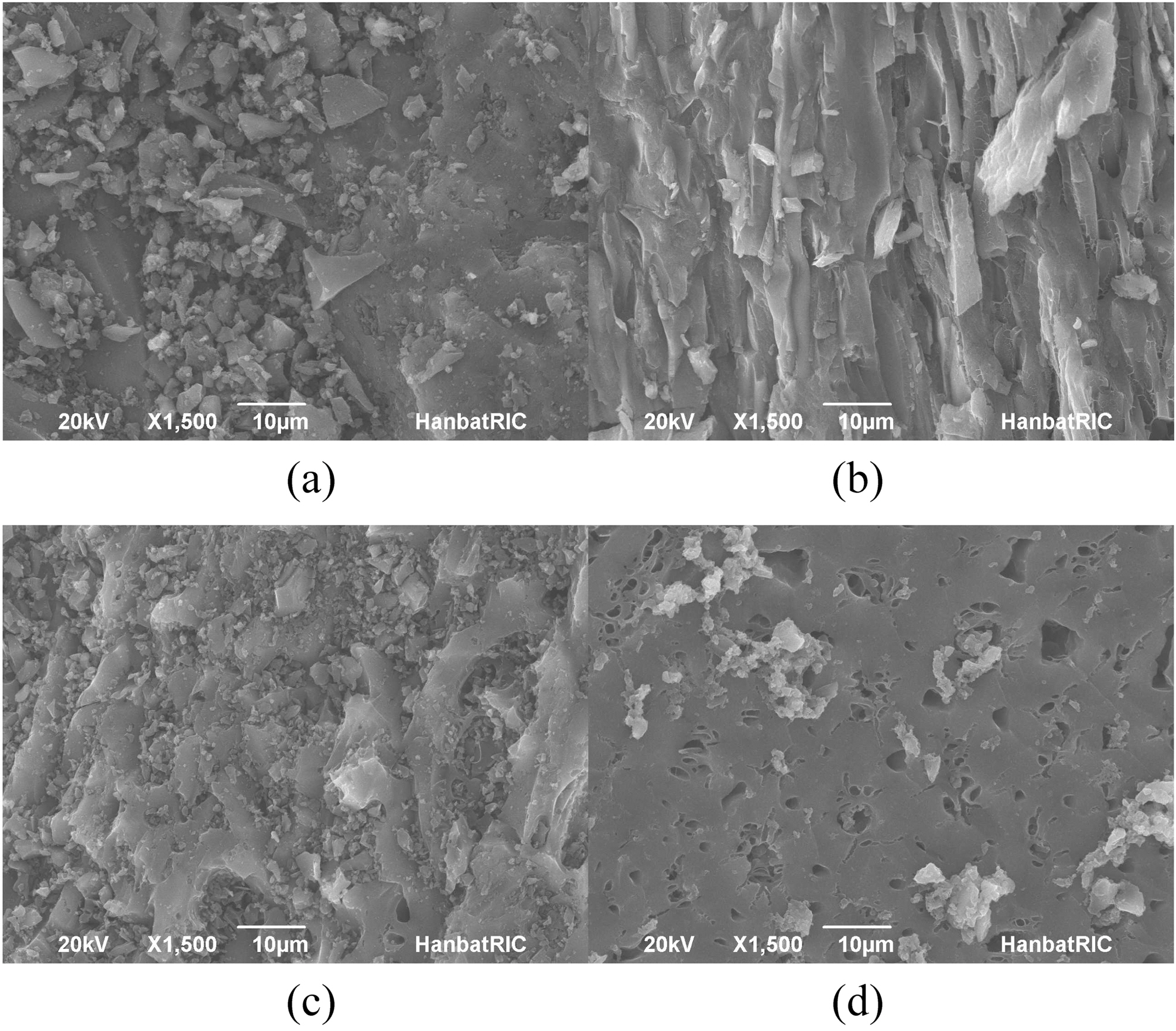
The SEM images of the resulting AC-containing PVA/PAAc composite hydrogels are presented in Fig. 1. It is clear that AC did exist in the PVA/PAAc hydrogel network to form a new composite system: activated carbon in hydrogel, although some AC was aggregated. As can be seen from Fig. 1, AC was uniformly distributed in the hydrogel matrix and retained the similar surface structure as AC itself.
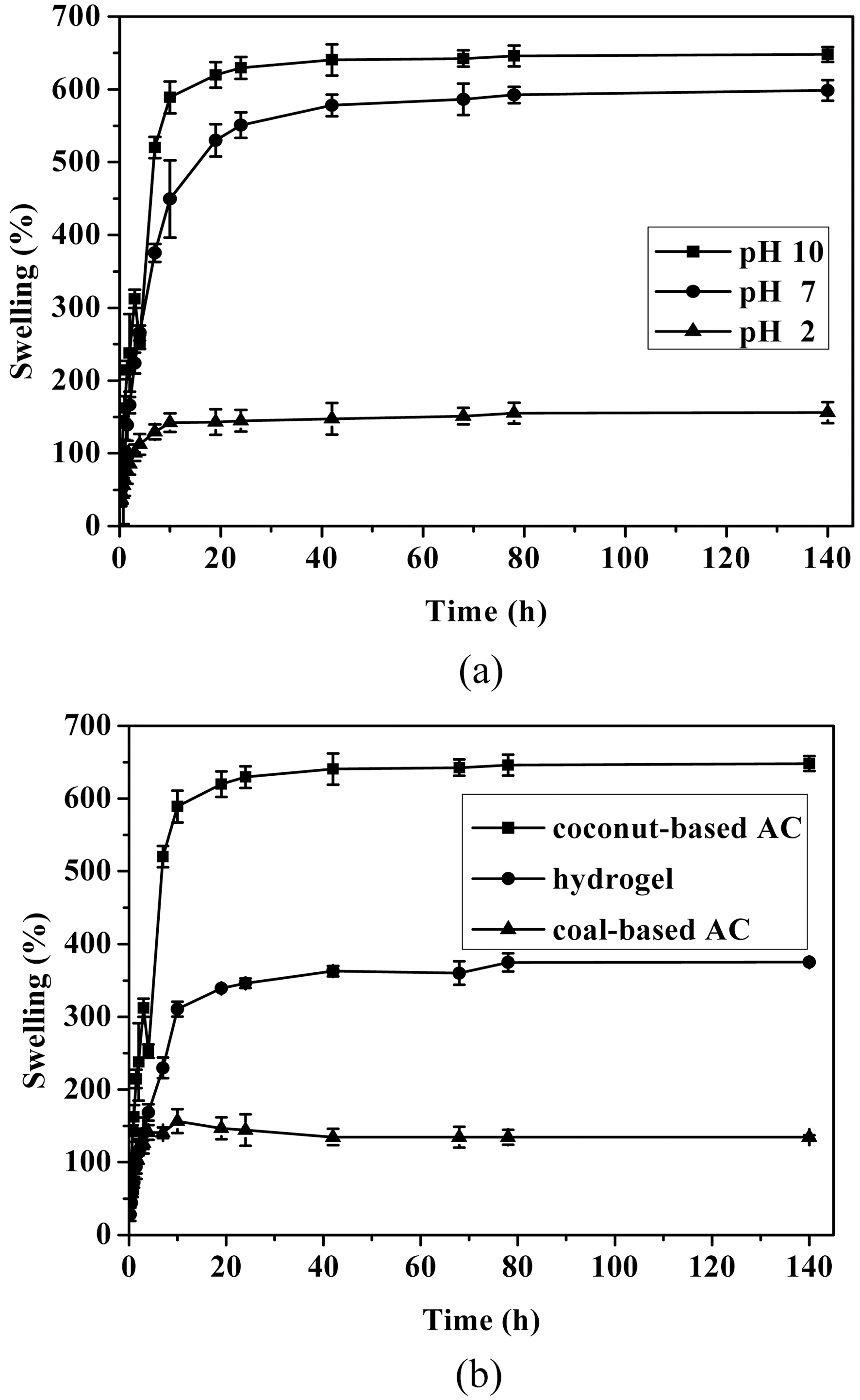
The effect of pH on the swelling of AC-containing PVA/PAAc composite hydrogels is presented in Fig. 2. Even
[Table 2.] Textural properties of activated carbons
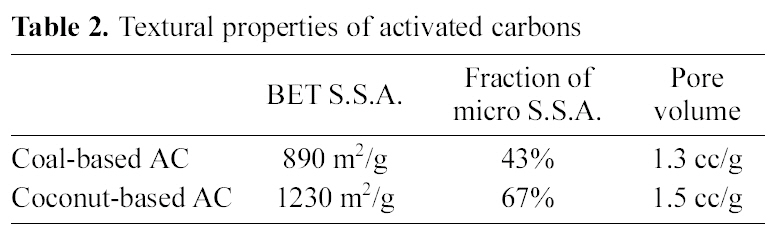
Textural properties of activated carbons
though they swelled in all cases, the swelling behavior was quite different. In cases of pH 2 and pH 7, the degrees of swelling were about 150 and 550%, respectively. Among three cases, the degree of swelling was highest in the case of releasing coomassie brilliant blue at pH 10. The reason of this pH-sensitive behavior is that the PAAc is ionizable hydrophilic polymers. PAAc network is able to swell in water. Its swelling behavior is greatly pH-dependent due to the ionization/deionization of the carboxylic acid group. At low pH, the -COOH groups are not ionized and keep the network in its collapsed state. At higher pH, the -COO- groups repel each other, leading to the swelling of PAAc network. The swelling behavior of AC-containing PVA/PAAc composite hydrogels was quite different depending on the kind of AC incorporated. The degrees of swelling of the coal-based ACFig
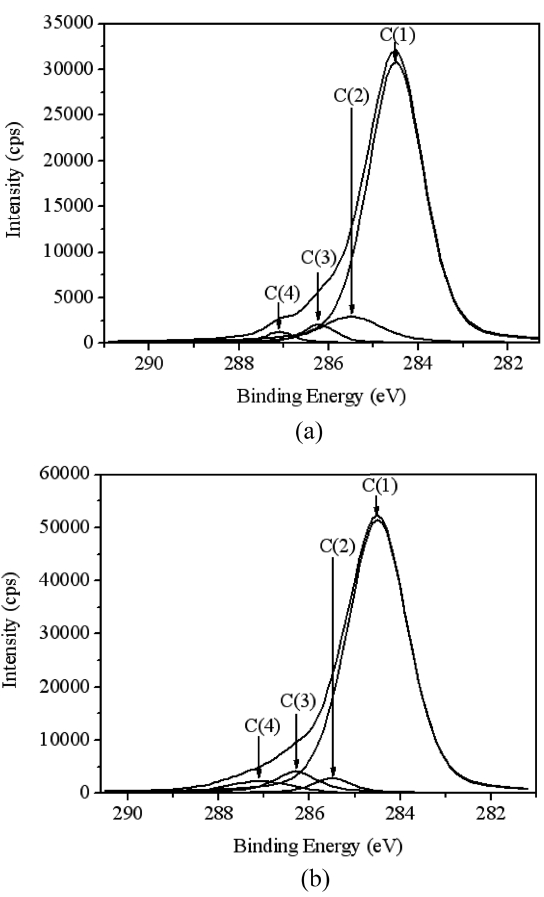
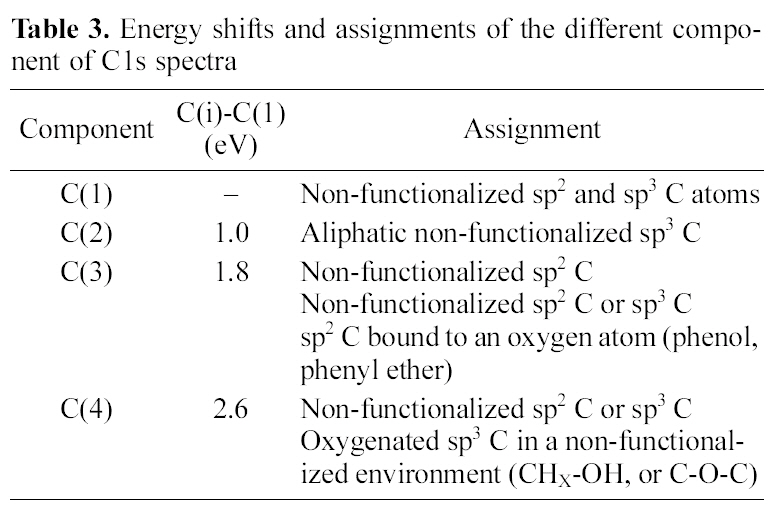
[Table 3.] Energy shifts and assignments of the different component of C1s spectra
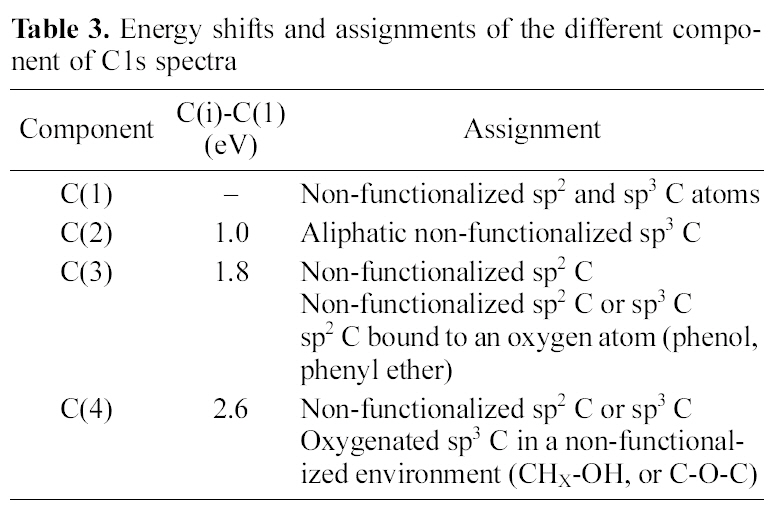
Energy shifts and assignments of the different component of C1s spectra
containing composite hydrogel and the control hydrogel were about 150 and 350%, respectively. Among three cases, the degree of swelling was highest for the composite hydrogel containing coconut-based AC. This result seems to come from the surface nature of AC depending on the kind of AC.
In order to investigate the reasons which resulted the difference of swelling ratio in both cases, the chemical structure of AC surface was studied by using XPS as shown in Fig. 3 and Table. 3 and 4. Regarding the deconvolution of C1s peak of two samples, it was sure that the surface of coconutbased AC was more hydrophilic through acid treatment,because it had more oxygenated sp3 representing hydrophilic functional groups such as CHx-OH or C-O-C. The increased hydrophilicity of AC is believed to play an important role in the higher swelling of the acid treated coconut-based ACcontaining PVA/PAAc composite hydro-gel.
3.4. Surface morphological change of composite hydrogel by pH variation
The effect of pH on surface morphology was investigated using SEM as shown in Fig. 4. In case of immersing the composite hydrogel in the buffer solution of pH 2, the composite hydrogel did not show the swollen surface structure. On the other hand, the composite hydrogel swollen well in the buffer solution of pH 10 showed the expanded surface structure. These morphological results accord with the results shown in Fig. 2.
[Table 4.] C1s peak parameters of ACs
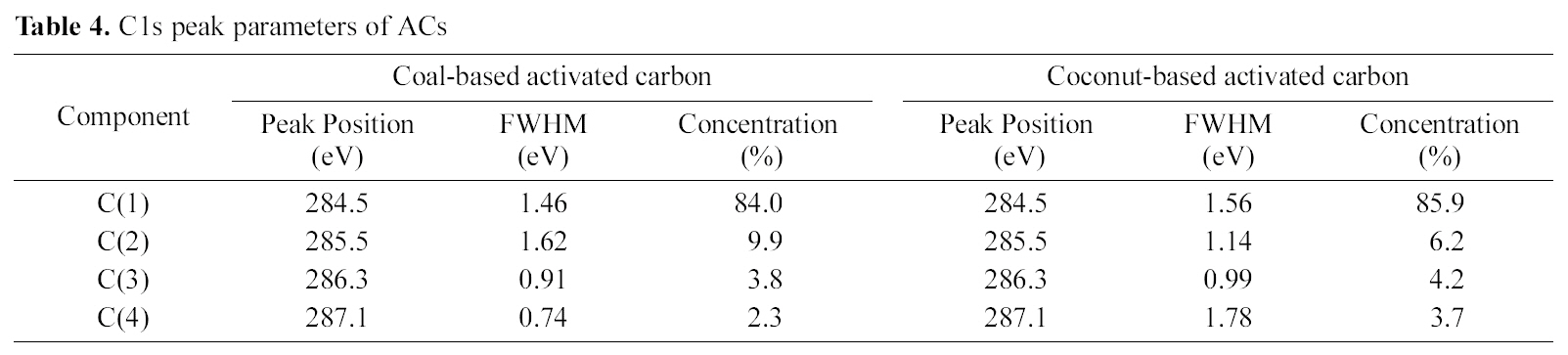
C1s peak parameters of ACs
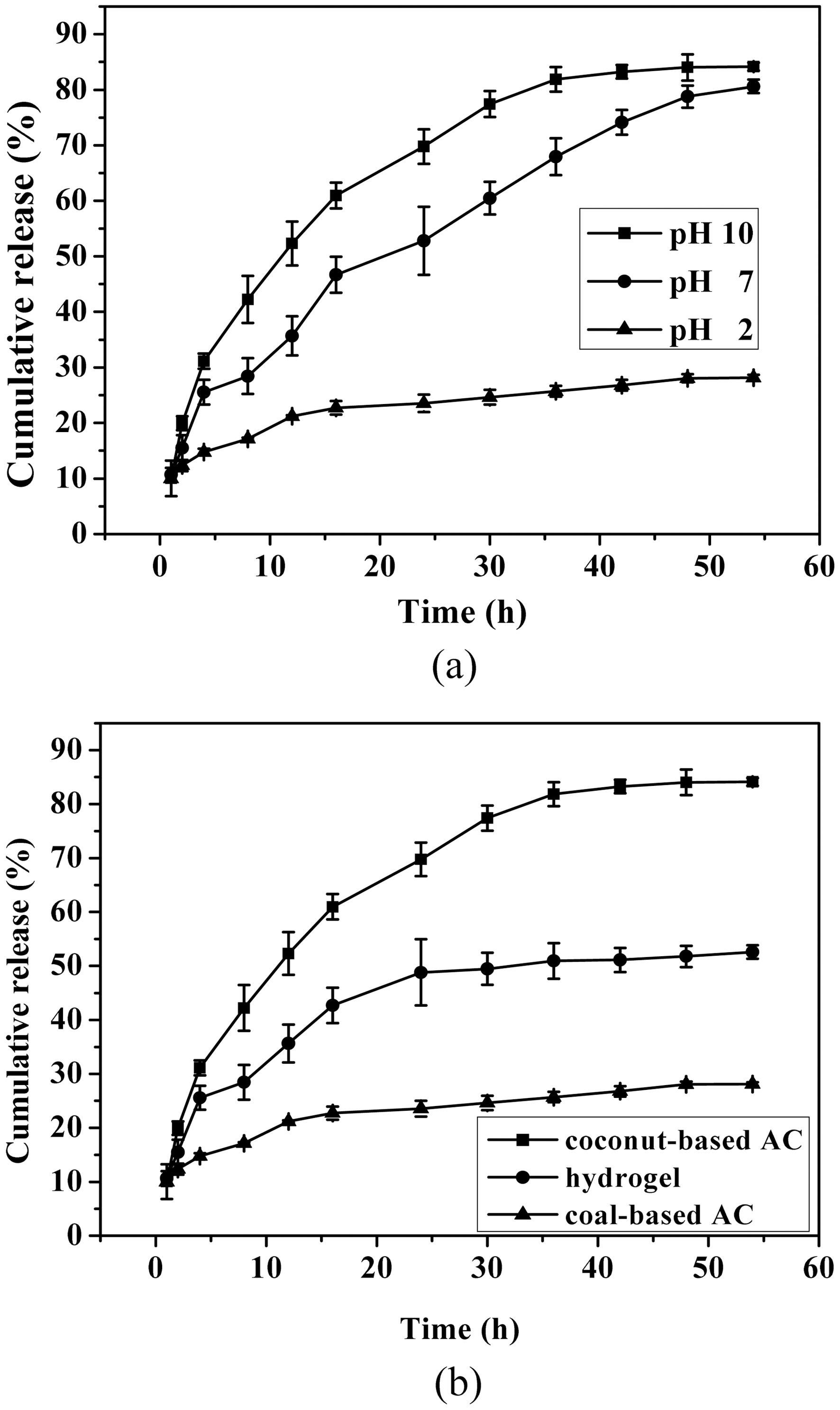
Coomassie brilliant blue was used as a model drug which was loaded in the AC reservoir. The drug release profile from AC-containing PVA/PAAc composite hydrogels is shown
in Fig. 5. The release amount of drug from the AC-containing PVA/PAAc composite hydrogel was increased rapidly at pH 10 due to an extensive swelling of hydrogel matrix. The pH-responsive hydrogel matrix could control the release of drug depending on the degree of swelling. The drug release behavior of AC-containing PVA/PAAc composite hydrogel was quite different depending on the AC used. Degrees of drug release from the coal-based AC-containing composite hydrogel and the control hydrogel at pH 10 were about 55 and 90%, respectively. Among three cases, the degree of releasing coomassie brilliant blue was highest at pH 10 for the coconut-based AC-containing PVA/PAAc composite hydrogel due to the extensively developed pore structure of hydrogel and the hydrophilic surface nature of AC.
The pH-sensitive PVA/PAAc composite hydrogels containing two different kinds of activated carbons were prepared. The drug was loaded efficiently due to the highly developed pore structure of AC. In case of the swelling, the coconutbased AC-containing PVA/PAAc composite hydrogel showed the highest swelling at pH 10, while the coal-based AC-containing PVA/PAAc composite hydrogel showed the lowest swelling. The higher release from the coconut-based ACcontaining PVA/PAAc composite hydrogel was due to the extensively developed pore sutructure of hydrogel at pH 10 and the hydrophilic surface nature of AC. The AC-containing composite hydrogel was a useful design to control the release of drug easily by varying both the kind of AC and the pH.