Advanced oxidation process in the presence of semiconductor catalyst is an effective method to degrade lot of organic, inorganic, dyeing compounds in mild condition of temperature, pressure, in the presence of UV radiation and in the presence or absence of catalyst. The photomechanism in simple, can be explained as that under the influence of UV, electron is promoted from valence band to conduction band of semiconductor oxides, thus generating a hole in valence band. The activated electron can react with oxidizing agent yielding a reduced product and the generated hole can react with reductant to produce an oxidized product [1].
Photocatalytic degradation uses usually TiO2 as slurry or immobilized or doped with metal/non-metals. Titanium di oxide has been widely utilized as a photocatalyst for generating electrons (e-) and holes (h+), thereby inducing reductive and oxidative reactions, respectively. The electrons and holes can be excited through UV light irradiation to overcome the band gap energy. Accordingly O2- and OH. radical ions are produced and mineralize pollutants [2]. In order to lower the band gap energy, the structure of the catalyst is modified. Modification could be done by doping, immobilization, structure modification and sensitization. Previous works have been done by doping with metals and non-metal dopants. First non-metal doped TiO2 was described in 1986 by Sato,
An organic compound which can be degraded using this mechanism is quinol. Quinol is used in the electronic industry for washing purposes and also a major component in photographic developers. They are also used as skin whitening agents in dermatological preparations. The effluents from textile industry are also the major pollutants for several decades. Blue FFS acid dye which is used in textile industry for dying of silk is a pollutant. It has been estimated that approximately 15% of textile dyes are lost in waste streams during manufacturing and processing operations. Many organic compounds are decomposed in aqueous solution in the presence of titanium dioxide powder illuminated with UV light. Due to the high concentration of organics in the effluents and the higher stability of modern synthetic dyes, the conventional biological treatment methods are ineffective for the complete color removal and degradation of organics and dyes Other conventional methods of color removal from an aqueous medium include techniques like coagulation, filtration, adsorption by activated carbon and treatment with ozone. These techniques require downstream processing units which add to the cost of degradation process.
The aim of the study is to examine the decomposition of Quinol and Blue FFS acid dye by photodegradation in the presence of UV light using suspended TiO2 with and without Boron-Nitrogen doping in an annular photocatalytic reactor with UV source of 250 W. Many authors have studied the performance of the metal doped catalyst under visible light [6]; hence experiments have been done to understand the decomposition under UV illumination. Industrial waste streams can be discharged with wide range of concentration and since photocatalysis is a surface phenomenon, the photocatalyst performance can be highly predisposed by the concentration of the stream, the nature of the compound, and its ability to absorb onto the photocatalyst surface. In acidic or caustic conditions, the surface of TiO2 can respectively become positively or negatively charged. This property is typically influenced by the compound concentration and photocatalytic reaction times. Hence the operational parameters like concentration of the compound, time of irradiation have also been optimized.
All the chemicals used were of analytical grade and the water used was distilled water. Quinol (CDH), TiO2(CDH), 4-amino antipyrine (Ranbaxy fine chemicals limited), Potassium ferricyanide (CDH), Ammonium chloride (CDH), Ammonia solution (SRL), Blue FFS acid, Triton x-100 (SRL), Titanium isopropoxide (SRL), Boric acid (SRL), Urea (Ranbaxy fine chemicals limited )were used.
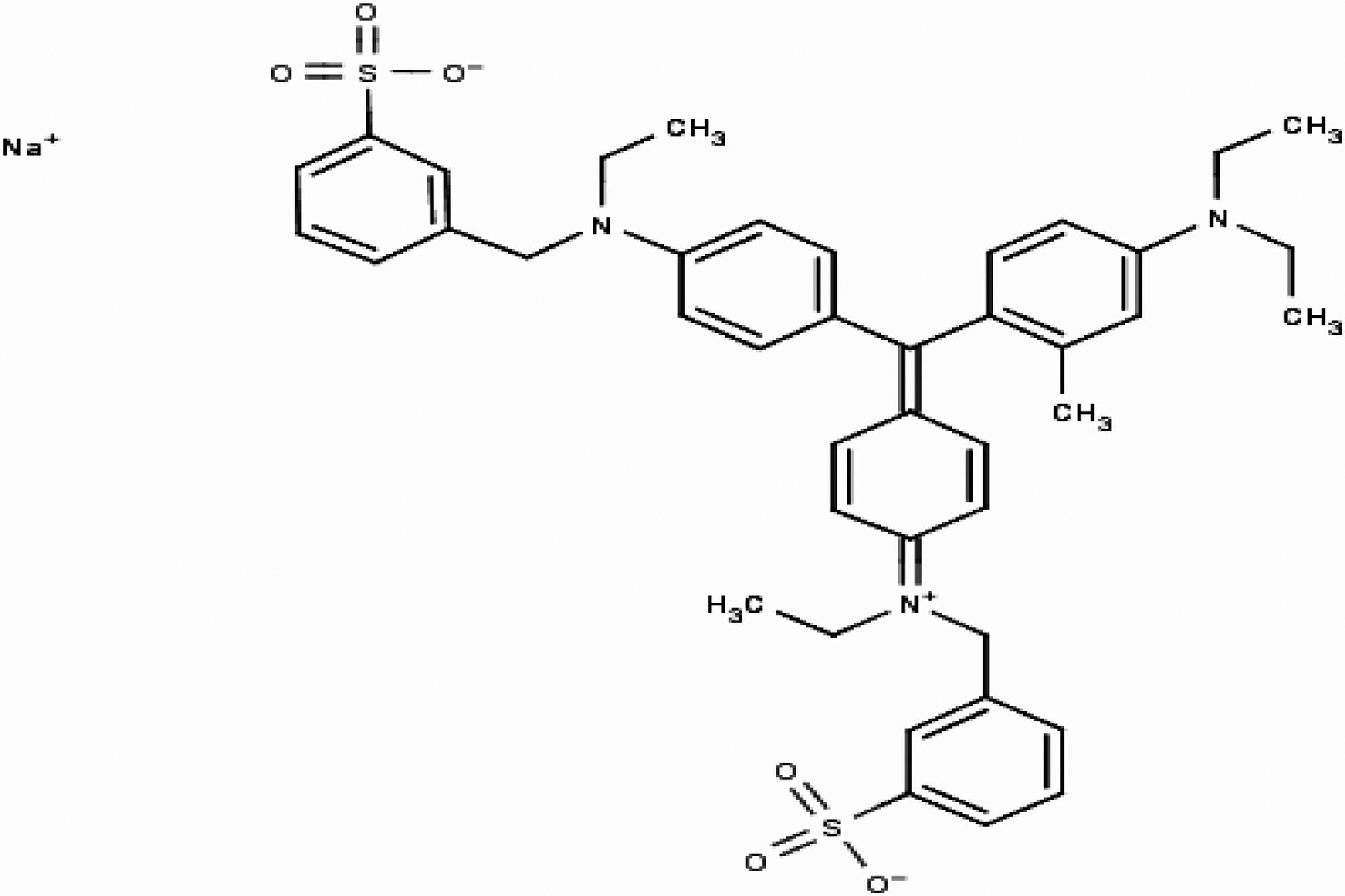
Quinol is also known as hydroquinone or p-benzenediol. It has the chemical formula of C6H6O2. It is an off-white powder or needle-like crystals with melting point of 172℃. Blue FFS acid is generally referred to as Acid blue 15 with chemical formula C42H46N3NaO6S2 and molecular weight of 775.95. Commercially with CI number 42665, Acid blue 15 is also referred to as Colocid Brilliant Blue FF, Dinacid Brilliant Blue B, Lecotan Blue AL, Monacid Coomassie Blue FF, Raviramine Blue BS whose chemical structure is given in Fig. 1.
2.2. Photocatalytic degradation
For experiments under UV light, stock solutions of the dye
and quinol with desired concentration were prepared with distilled water. An immersion well photoreactor made of pyrex glass was used as a batch reactor. The UV fluorescent tube, which acts as light source was placed in the middle of the reactor vessel around which the cooling water was supplied continuously. UV light source of 250W UV lamp was used. Samples were collected at regular intervals and centrifuged at 3000 rpm for 15 min. In the case of quinol, photodegradation was monitored by forming a complex compound using 4-amino antipyrine and measuring the absorbance at 460 nm by using UV- Vis spectrophotometer. In the case of Blue FFS acid, photodegradation was monitored by measuring the absorbance at 562 nm by using UV- Vis spectrophotometer. Then the percentage of quinol and Blue FFS acid degradation was calculated by using the Eq. (1)
where,
C0 - Initial Concentration, mgL-1
C - Variable Concentration at time t, mgL-1
2.3. Preparation of B-N codoped TiO2
B-N codoped TiO2 was prepared by hydrothermal synthesis. 3.4 g Ti(OBu)4 and 0.72 g Triton x- 100 were added to 20 mL of ethanol under stirring. Then a solution of 3 ml saturated urea solution containing 0.12 g boric acid was added drop wise. After stirring for an hour, the mixture was autoclaved at 120℃ for 14 h and left to stand for cooling naturally. It was then washed with ethanol and then dried at 75℃ for 1 h and calcined at 450℃ for 1 h.
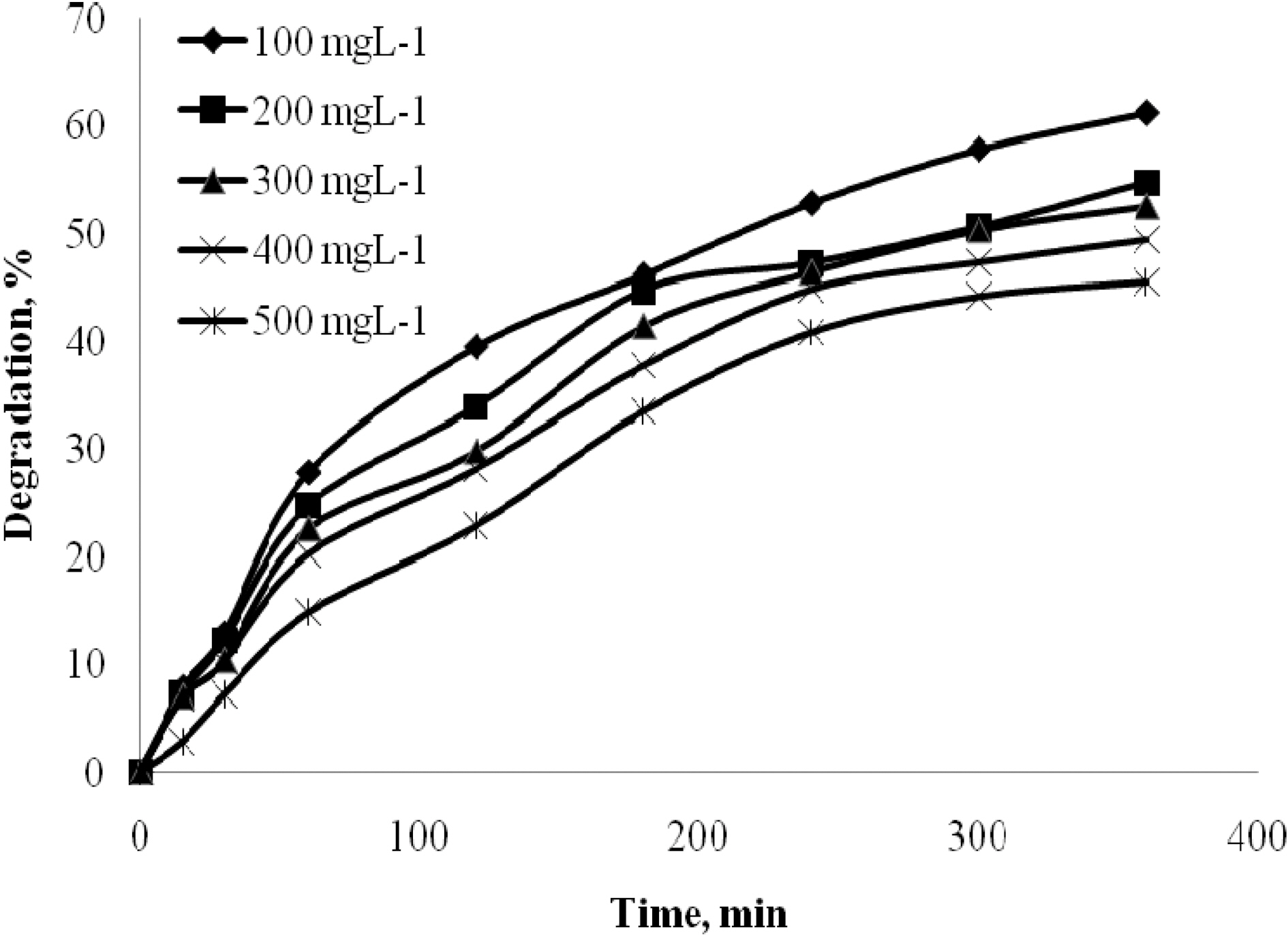
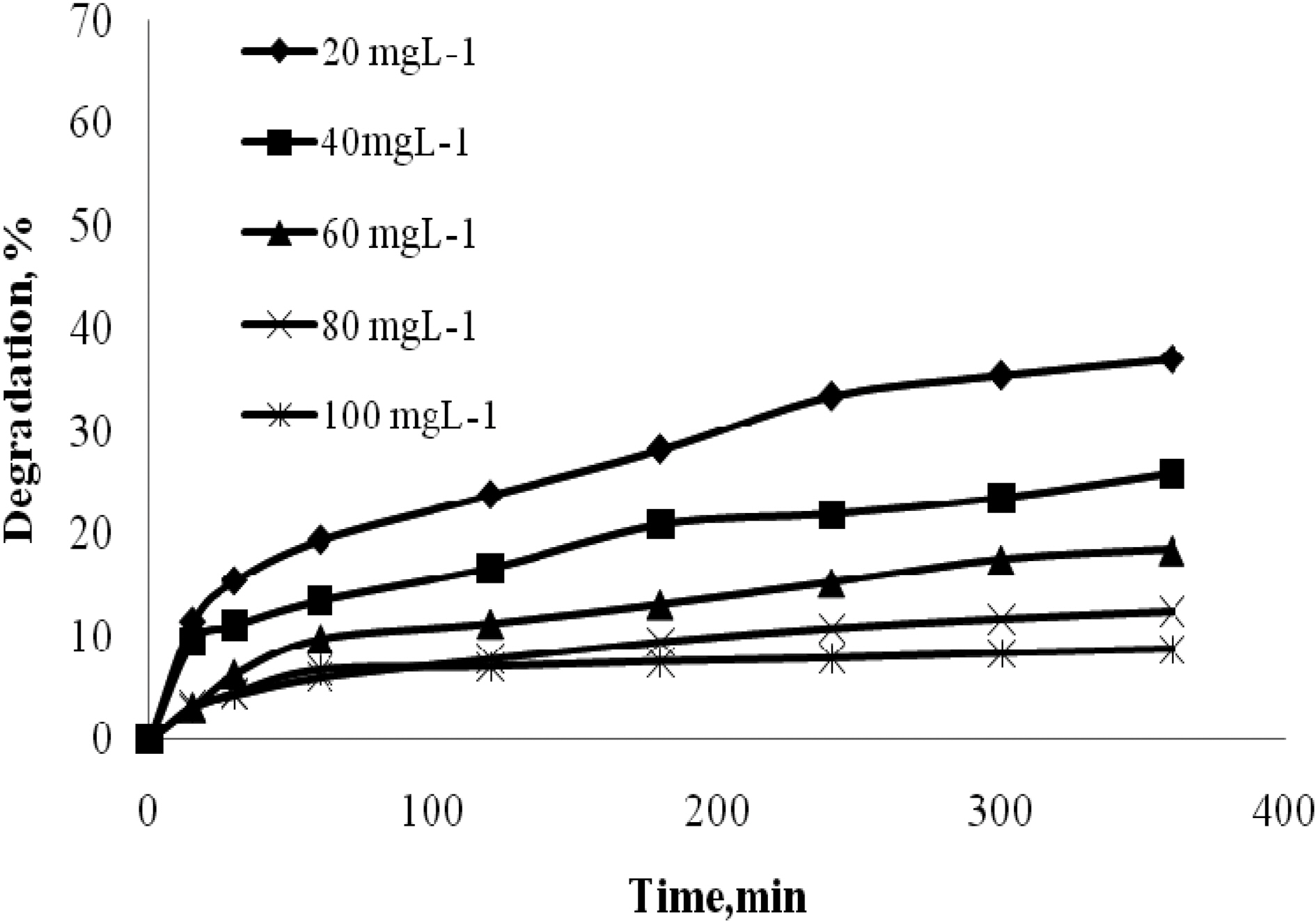
The effect of time was analyzed for quinol with different initial concentrations 100 to 500 mgL-1. The effect of time was analyzed for Blue FFS acid with different initial concentrations 20 to 100 mgL-1. Catalyst used was 2 gL-1 of TiO2. The natural pH was maintained for all the concentration and the UV light intensity was kept constant at 250W. Fig. 2 and Fig. 3 show that in the case of quinol and Blue FFS acid, the percentage degradation increases with increase in contact time. The OH· formed on the surface of TiO2 is constant, the relative number of OH· attacking the compound increases, and thus the photodegradation efficiency increases with time [7].
3.2 Effect of initial concentration
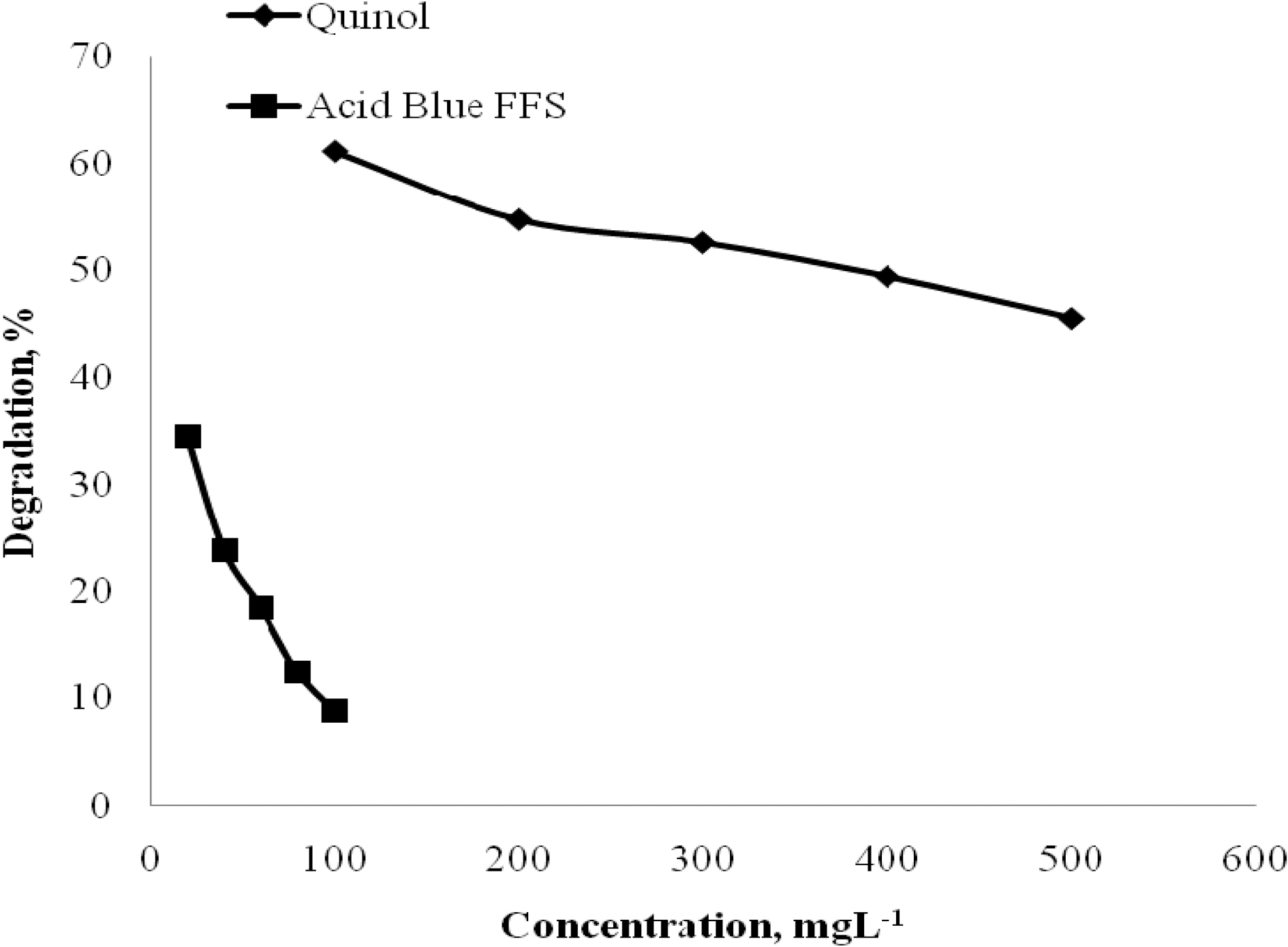
The effect of initial concentration of quinol on the
photodegradation rate was analysed by varying the initial concentration from 100 to 500 mgL-1. The effect of initial concentration of Blue FFS acid dye on the photodegradation rate was analysed by varying the initial concentration from 20 to 100 mgL-1. Catalyst used was 2 gL-1 of TiO2. Fig. 4 shows that percentage degradation decreases as the initial concentration of quinol and Blue FFS dye increases. As the concentration increases, the number of photons that reaches the catalyst surface decreases. Hence, the number of catalyst molecules that is excited reduced. The rate of formation of holes, hydroxyl radicals and supra oxide ions which greatly depends on the excitation of the catalyst molecules decreases and hence there is decrease in the photodegradation rate. The amount of the molecules that attached to the active sites of the catalyst also gets limited because the catalyst surface area remains the same for all concentration of the compounds. At higher concentration a competition for the molecules to get attached to the surface increases and hence degradation decreases with increase in concentration [8].
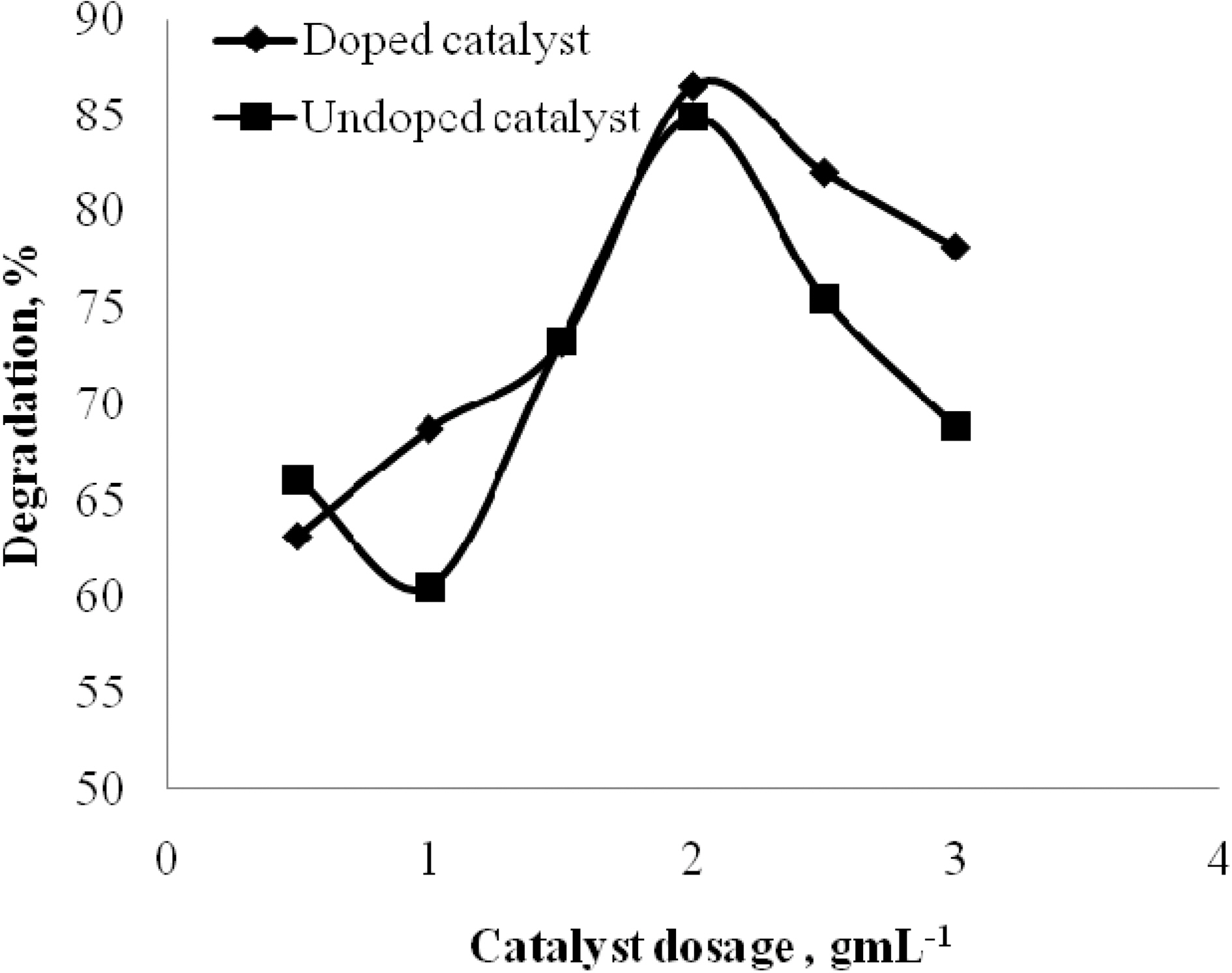
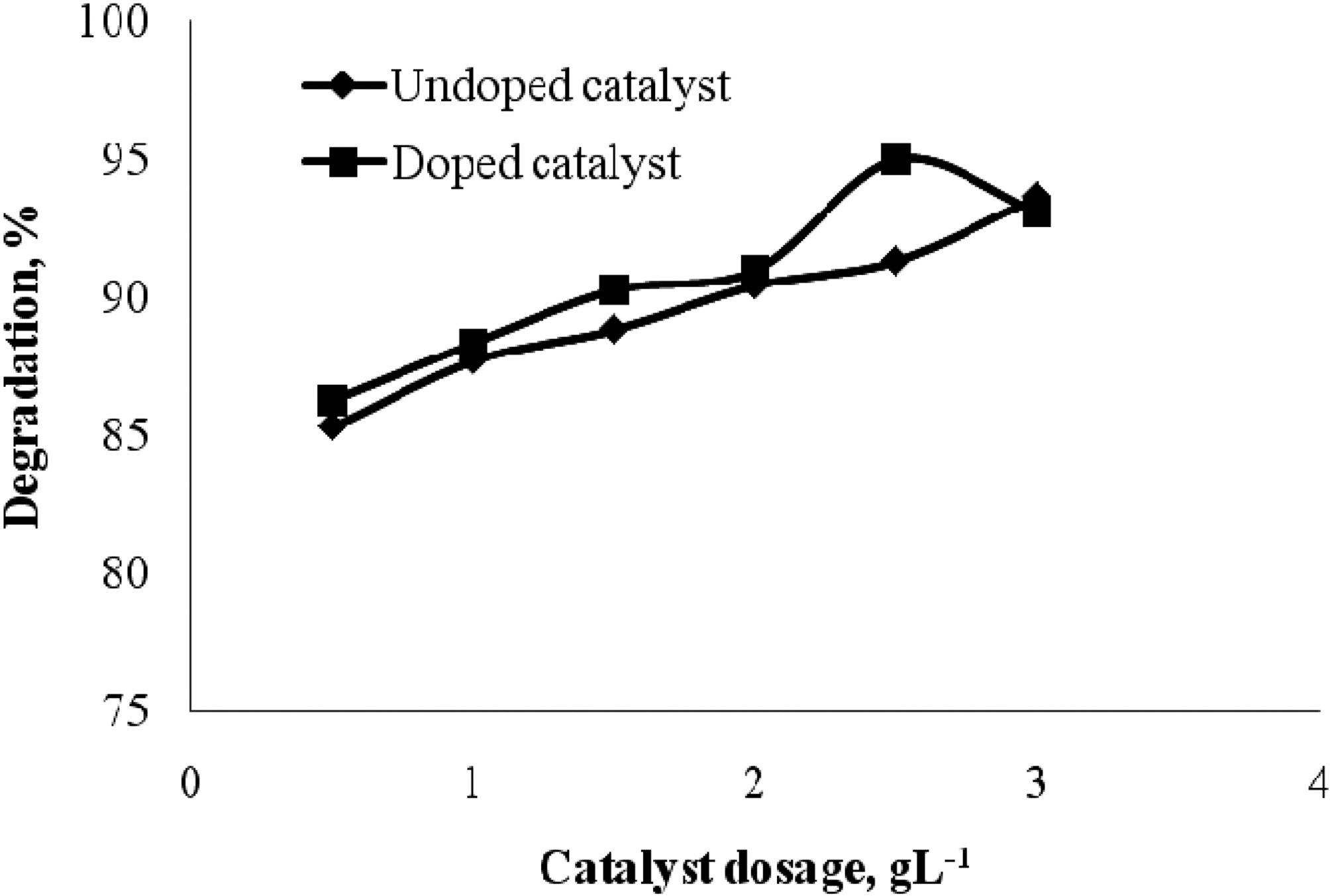
3.3. Effect of Doped and undoped catalyst
The effect of doped and undoped catalyst on the photocatalytic degradation of quinol and Blue ffs acid was studied by varying the amount of doped TiO2 and undoped TiO2 from 0 to 3 gL-1 and the degradation profile is shown in Fig. 5 and Fig. 6. From the figure, it is vivid that the percentage degradation by using B-N codoped TiO2 is higher than by using undoped TiO2 for both quinol and Blue FFS acid dye. The series of experiments performed by varying the amount of TiO2 from 0 to 3 gL-1 shows that the percentage degradation of quinol is found to increase linearly upto 2 gL-1 above this amount, increased turbidity of the solution reduced the light transmission through the
solution, while below this level, it is assumed that the catalyst surface and the absorption of light by TiO2 were limiting. The observed enhancement in the degradation is probably due to the increased number of catalytic sites on TiO2. A further increase in the amount of TiO2 decreases the percentage degradation of quinol. In the case of Blue FFS acid dye the percentage degradation is found to increase significantly in the presence of catalyst [6]. The percentage degradation of Blue FFS acid dye is found to increase constantly from 0.5 to 3 gL-1.
The doped catalyst has better performance over the undoped catalyst. The possible reasons could be, in a Boron-Nitrogen doped catalyst, there is reduction in band gap energy, irradiation with UV light excites electrons in both the valence band and the impurity energy levels and finally, nitrogen may be doped in the oxygen deficient sites which help in blocking re-oxidation [9,10].
The photodegradation of quinol and Blue FFS acid was studied for various parameters like time, initial concentration, dosage of catalyst, dosage of doped catalyst. From the experimental data the following conclusions are made. The photodegradation of quinol and Blue FFS acid increases with increase in time. The photodegradation of quinol and Blue FFS acid decreases with increase in initial concentration. The present work illustrates that photocatalysis is an effective and sustainable method for the decomposition of organic compounds and dyes. The degradation effiency using photocatalyst is higher compared to that of photolysis. The B-N- codoped TiO2 showed higher photodegradation effiency than undoped TiO2.