There is a growing interest in the use of magnetic nanosized particles in various biomedical applications. In particular, iron-oxide nanoparticles have been used as magneticresonance imaging contrast agents for the targeted drug delivery [1-3]. Recently, the fabrication and magnetic characterization of iron-oxide (γ-Fe2O3) nanoparticles precipitated inside the alginate beads have been reported [4].Hydrogels are promising materials used for biomedical applications due to their bio-similarity, aqueous environment,porous structure, and facility of conjugation with various biological macromolecules [5-9]. Alginate is a hydrogelforming polysaccharide consisting of (1-4) linked β-Dmannuronate (M) and its C-5 epimer α-L-guluronate (G) residues [10]. One of the attractive properties of alginate is the versatility of gel formation simply induced by various divalent cations. Alginate hydrogel beads cross-linked by Ca2+ are the most typical one and have been widely utilized in tissue engineering and drug delivery [11-14]. The other attractive property of alginate is the pH-dependent solubility due to the carboxyl groups in the backbone. Therefore, alginate has the properties of shrinking at lower pH and getting dissolved at higher pH [15]. The alginate hydrogel beads could protect the acid-sensitive drug from the gastric fluid and the drug could be delivered safely to the intestine [16-17].
Activated carbon (AC) is one of the most effective adsorbents for several organic compounds because of their extended surface area, high adsorption capacity, micro porous structure, and specific surface activity [18-19]. Coconut shell-based AC is an effective candidate for drug adsorbent because it has high specific surface area and well-developed pore structure.
Vitamin B12 (VB12), a model drug, combines with a substance called gastric intrinsic factor (IF). This complex can then be absorbed by the intestinal tract. IF is secreted by the stomach lining and it tightly binds VB12 and helps it pass through the intestinal lining and into the blood. VB12 is needed for normal nerve cell activity, DNA replication, and blood cell production. However, VB12 deficiency can potentially cause severe and irreversible damage, especially to the brain and nervous system. At levels only slightly lower than normal, a range of symptoms such as fatigue, depression,and poor memory may be experienced.
The objective of this study is to protect the loaded drugs from the gastric condition and to deliver effectively to the target intestinal area by using the alginate/AC composite beads loaded with
AC was purchased from Dong Yang Tanso Co. (Korea). Alginic acid sodium salt (from brown algae, viscosity of 2% solution at 25℃ : ~250 cps), γ-Fe2O3, calcium chloride (CaCl2), PVA, glutaraldehyde (GA), span 80, and n-hexane were obtained from Sigma Chemical Company. VB12, the model drug, was purchased from Samchun Chemical.
2.2. Preparation of γ -Fe2O3 microcapsules
The γ-Fe2O3 microcapsules having shell of PVA and core of γ-Fe2O3 were synthesized by emulsion polymerization method. A mixed aqueous solution of PVA (Mw 31,000-50,000, 10 wt%, 20 ml) and γ-Fe2O3 (0.2 g) was slowly added to n-hexane as oil phase (150 ml) containing span 80 as emulsifier (7 ml) with stirring at 1000 rpm for 1 h at 50 to form the water-in-oil emulsion. GA as crosslinking agent (25 wt%, 1 ml) was slowly added to the emulsion with stirring at 1300 rpm for 3 h at 50℃. The γ-Fe2O3 microcapsules were formed and purified by multiple washing with distilled water and petroleum ether, respectively, followed by solvent evaporation under vacuum at 50℃.
2.3. Preparation of vitamin B12-loaded activated carbon
AC was pretreated with washing at 100℃ for 2 h using mixed acids of sulfuric (35 wt%) and nitric (20 wt%) acids with weight ratio of 1:1. The treated AC was loaded with VB12 by immersing in the aqueous solution of VB12.
2.4. Preparation of alginate/AC composite hydrogel magnetic beads
The microorganism solution was prepared by mixing the aqueous solution of sodium alginate (1.5 wt%) and
2.5. Characterization of alginate/AC composite hydrogel magnetic beads
2.5.1. Morphology
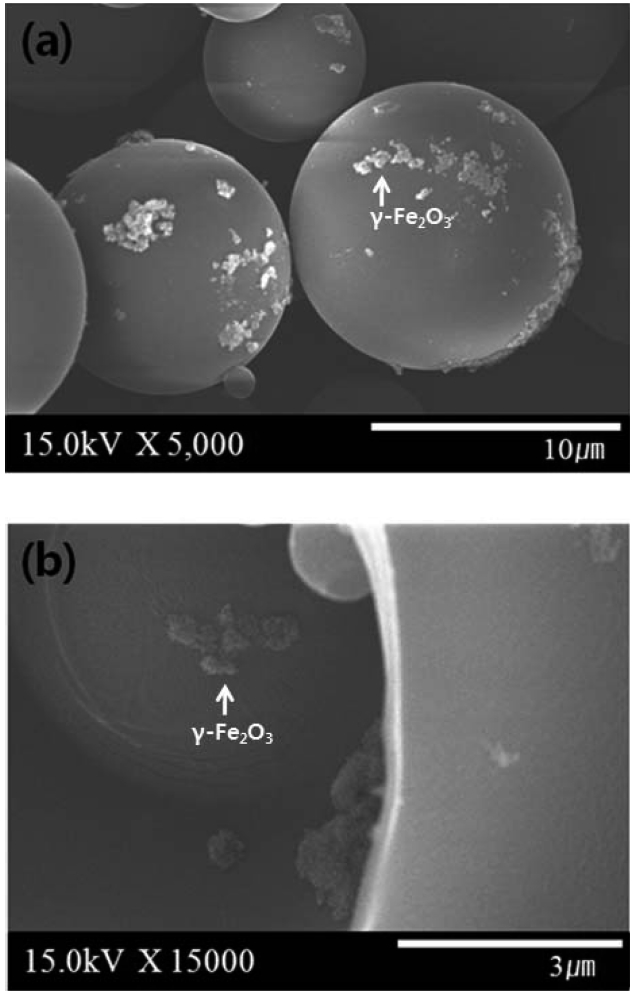
The morphology of γ-Fe2O3 microcapsules was monitored by the optical microscope (Olympus, CH-2). The surface morphology of alginate/AC composite magnetic beads andγ-Fe2O3 microcapsules was evaluated by JSM-7500F (Jeol, Japan) scanning electron microscopy. As a pretreatment, the composite beads were vacuumed up to 10-3 Pa and sputtered using Pt. γ-Fe2O3 microcapsules were sputtered using Os.
2.5.2. Magnetic characteristic
The magnetization curves of the dried alginate/AC composite hydrogel magnetic beads were determined with 2900-02 AGIM vibrating sample magnetomer (PMC Co.).
2.5.3. Drug release behavior
The release of VB12 from alginate/AC composite beads was carried out in each of the various buffer media (pH 2, 7, and 10) at 25 ± 1℃. Accurately weighed beads were placed in vials containing 25 ml of the buffer medium and maintained in a thermostat bath. At each predetermined time interval, 0.5 ml of buffer was collected from the release medium. The released amounts of VB12 were measured by UV/VIS spectrophotometer (Optizen 2120 UV, Mecasus, Korea) at 361 nm. The cumulative release was calculated according to the following equation:
Cumulative amount released (%) = (Mt/M)× 100
where Mt is the amount of drug released from the composite beads at time t and M is the amount of VB12 pre-loaded in AC. All the release experiments were carried out three times.
2.5.4. Survivability of Lactobacillus lamnosers
Survivability of
3.1. Morphology of γ -Fe2O3 microcapsules
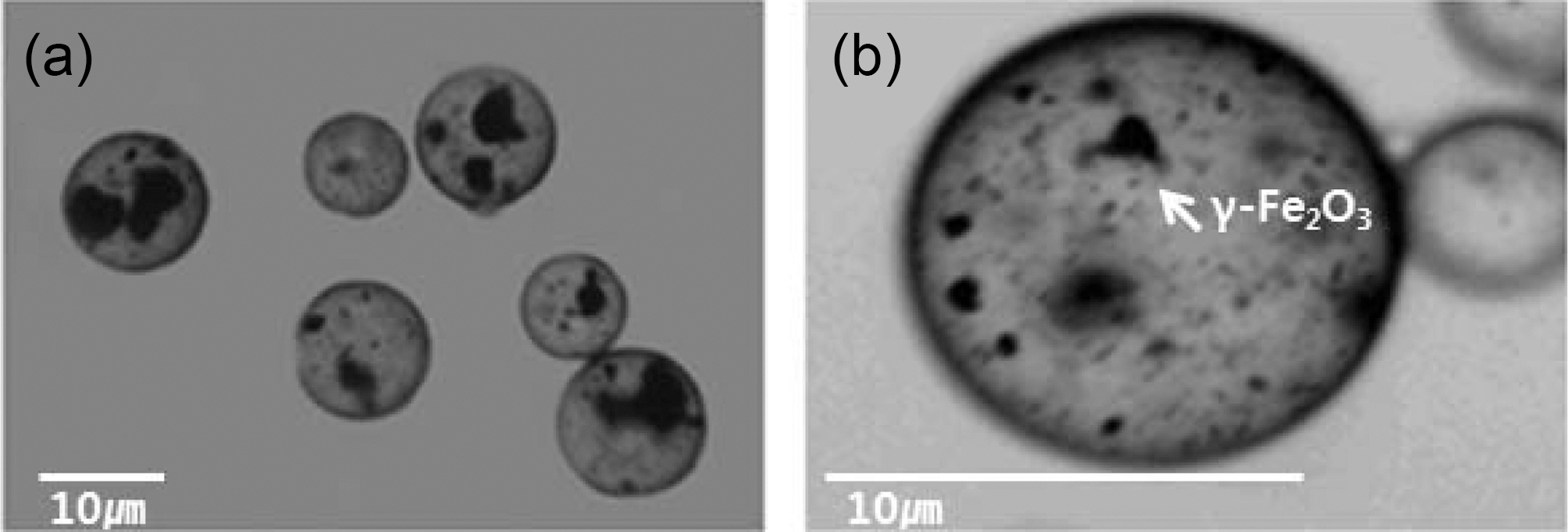
The optical microscope images of the γ-Fe2O3 microcapsules are presented in Fig. 1. Within each microcapsule, the black-colored γ-Fe2O3 particles are uniformly embedded in the microcapsules although some agglomeration was observed. Fig.
2 shows the SEM microphotographs of γ-Fe2O3 microcapsules. γ-Fe2O3 microcapsules were spherical with a their mean diameter of about 10 ㎛. The fractured microcapsules showed the γ-Fe2O3 contained in the core of microcapsules.
3.2. Magnetic characteristics of alginate/AC composite hydrogel magnetic beads
The composite hydrogel beads were spherical and blackcolored due to the presence of AC and γ-Fe2O3 inside the alginate matrix. AC and γ-Fe2O3 microcapsules were dispersed well in the composite hydrogel beads. The beads had the wet size of 3.00 mm and the dry size of 1.70 mm. Fig. 3 showed the magnetic properties of composite hydrogel beads. The saturation magnetization of composite hydrogel beads reached to 0.01 emu and the ferromagnetic nature of composite hydrogel
beads. The saturation magnetization of composite hydrogel beads reached to 0.01 emu and the ferromagnetic nature of composite hydrogel beads was seen from the hysteresis curve.The ferromagnetic properties are known useful in magnetic resonance imaging and therapeutic agent because of their inherent biocompatibility and relative stability of chemical and magnetic characteristics in ambient conditions [20]. The alginate/AC composite hydrogel magnetic beads in aqueous fluid were easily attracted under an external magnetic field as shown in Fig. 4. The composite beads exhibited a clear magnetic response because γ-Fe2O3 particles were immobilized uniformly in the composite beads. The composite hydrogel beads could be easily moved in the aqueous phase with an external magnet.
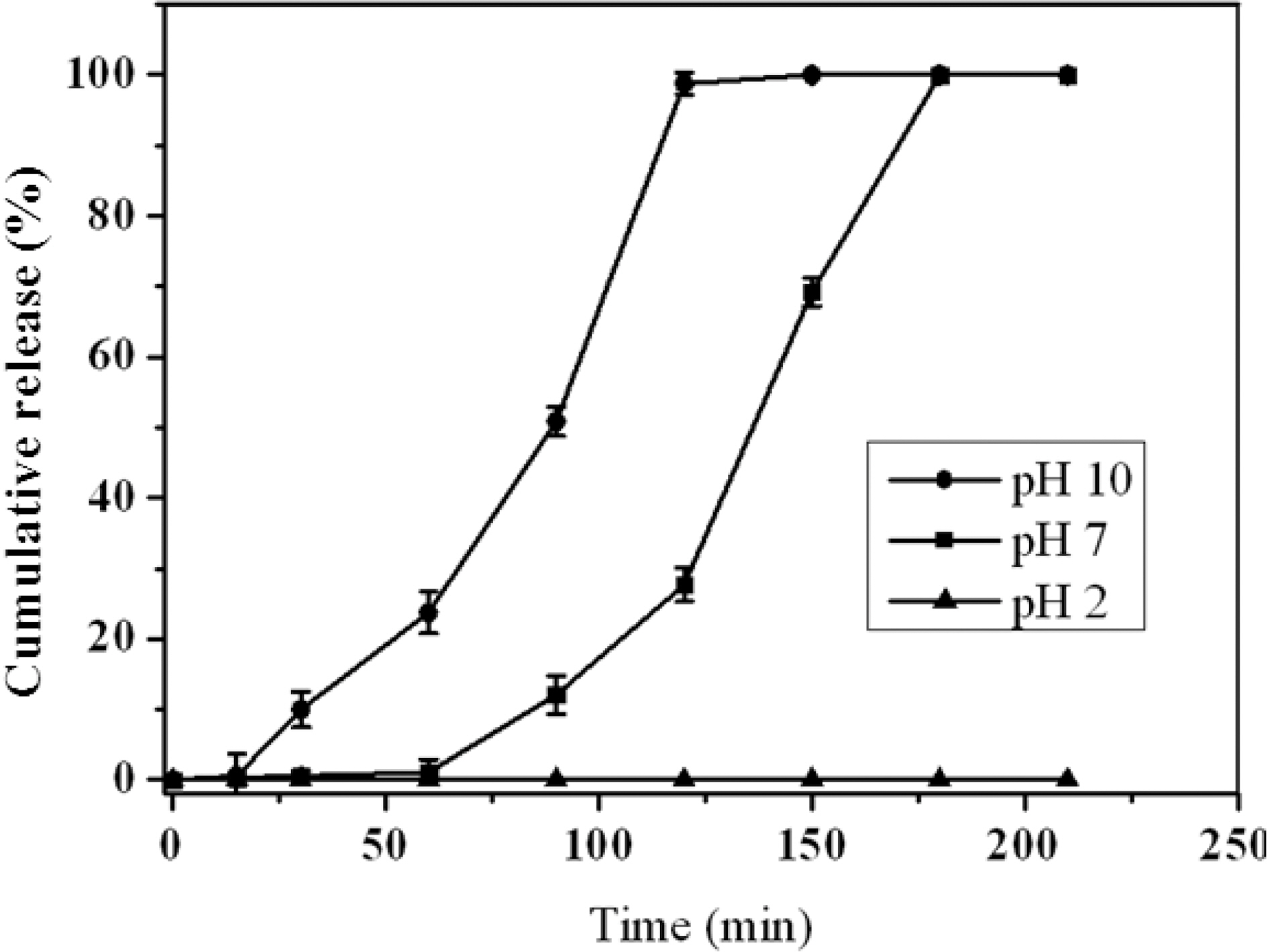
The release behavior was investigated for the alginate/AC composite beads containing VB12 in several different buffer media at room temperature. The composite beads containing VB12 were placed in the buffer media from pH 2 to 10 and the released amounts were measured depending on time. The pH-sensitive release behavior of the alginate/AC composite beads is well presented in Fig. 5. The released amount of drug from the composite beads increased as the pH increased into the basic condition due to the selective dissolution of alginate matrix. The alginate/AC composite beads could control the release of VB12 successfully depending on the degree of selective dissolution of alginate matrix by changing the pH of the release media. Therefore, the alginate/AC composite beads were able to protect the loaded drug effectively from the acidic gastric condition.
The effects of pH on the survivability and the protection of
The alginate/AC composite hydrogel beads containing γ-Fe2O3 microcapsules were prepared as a pH-sensitive drug delivery system to protect and deliver the loaded drug effectively. The AC and γ-Fe2O3 clusters were uniformly dispersed in the alginate hydrogel matrix. The alginate/AC composite hydrogel beads could be easy moved to the target by using external magnet due to the γ-Fe2O3 clusters in alginate matrix. VB12 was released from the composite beads with sustained and pH-sensitive manner. The drug was released fast in the basic condition based on the pH-sensitive solubility of alginate hydrogel. The